Abstract
The present study investigated the expression of microRNA-503 (miR-503) and its effect and mechanism of action on prostate cancer. Tumor tissues and tumor-adjacent tissues were collected from 20 patients with prostate cancer. TargetScan was used to predict the miRNA molecule that interacts with tumor protein D52 like 2 (TPD52L2). DU145 cells were transfected with a negative control, miR-503 mimic or miR-503 inhibitor. DU145 cells that had not undergone transfection were used as a control. Levels of miR-503 and TPD52L2 mRNA were determined using reverse transcription-quantitative polymerase chain reaction and the expression of TPD52L2 protein was measured using western blot analysis. The migration ability of DU145 cells was evaluated using a Transwell assay and cell proliferation was examined using an MTT assay. A flat plate colony formation test was conducted to examine the colony formation rate of DU145 cells. The current study demonstrated that TPD52L2 expression is increased while miR-503 expression is decreased in prostate cancer tissues. Overexpression of miR-503 inhibited the transcription and translation of TPD52L2 in DU145 cells and reduced cell migration, proliferation and colony formation. By contrast, inhibition of miR-503 expression increased the expression of TPD52L2 in DU145 cells and increased cell migration, proliferation and colony formation. The present study demonstrated that miR-503 is an oncogene that regulates the migration, proliferation and colony formation of prostate cancer cells by targeting the TPD52L2 gene. Thus, miR-503 has the potential to become a target for the molecular treatment and prognosis of prostate cancer in the future.
Keywords: prostate cancer, microRNA-503, tumor protein D52 like 2, DU145 cells
Introduction
Prostate cancer is a common malignant tumor in the urinary system of middle aged and elderly males and has a high incidence and mortality rate (1). The mechanisms of prostate cancer include the interaction between growth factors and epithelia-stroma (2), altered expression of tumor suppressor genes and oncogenes (3), DNA methylation (4) and androgen receptor mutations (5). Tumor protein D52 like 2 (TPD52L2), also known as TPD54 and D54, is a member of the tumor protein D52 (TPD52) family (6). The most important physiological function of the TPD52 family is its participation in the regulation of Ca2+-dependent vesicular transport processes (7). In addition, the overexpression of proteins in the TPD52 family in breast cancer (8) and hepatocellular carcinoma (9) suggests that the TPD52 family may serve an important role in the onset and development of tumors. A previous study on brain glioma cells demonstrated that knockout of TPD52L2 inhibits the growth and colony formation of cells by arresting cells in the G0/G1 phase (10). Similarly, it has been demonstrated that knockout of TPD52L2 in hepatic cells inhibits their growth (11).
microRNA (miR) is a type of small non-encoding single-strand RNA 18–24 nucleotides long that is present in eukaryote cells and viruses (12). It has been suggested that miRNA serves regulatory roles in the onset and development of prostate cancer, including the role of a tumor-suppressor (13). Furthermore, a previous study demonstrated that miR-15/miR-16 suppressed the activation of several oncogenes by inhibiting the transcription and expression of B-cell lymphoma-2, myeloid cell leukemia-1, cyclin D1 and Wnt family member 3A (14). It was also demonstrated that miR-23a downregulated the expression of glutaminase in prostate cancer cells, inhibited the catabolic metabolism of glutamine and inhibited tumor cell growth (15). miR-503 was first identified in human retinoblastoma tissues (16) and downregulation of miR-503 expression has been identified in liver, brain, lung, and colon tumors (17). miR-503 specifically targets B-cell lymphoma-2 and increases the sensitivity of A549/CDDP cells to cisplatin-induced apoptosis in small cell lung cancer cells (18). The present study investigated the expression of miR-503 and its effect on the proliferation and migration of prostate cancer cells.
Materials and methods
Patients
A total of 20 male patients with prostate cancer (mean age, 56.2 years) who had undergone radical prostatectomy at The People's Hospital of Rizhao (Shandong, China) between January 2016 and April 2016 were included in the present study. Prostate tissues were obtained from patients and pathologically confirmed as prostate cancer according to 2005 International Society of Urological Pathology (ISUP) consensus conference (19). None of the patients received radiotherapy or chemotherapy prior to surgery. Prostate cancer tissues and tumor-adjacent normal tissues (control) were removed and frozen at −80°C within 10 min following excision. All procedures were approved by the Ethics Committee of People's Hospital of Rizhao (Shandong, China) and written informed consent was obtained from all patients or patient families.
Bioinformatics
TargetScan (http://www.targetscan.org) was used to predict the miRNA molecule that interacts with TPD52L2 in order to understand the regulatory mechanism of TPD52L2 in prostate cancer.
Cells and cell transfection
DU145 cells (The Cell Bank of Type Culture Collection of Chinese Academy of Sciences, Shanghai, China) were cultured in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were divided into a DU145+miR-503 mimic group, a DU145+miR-503 inhibitor group, a negative control transfected (NC) group and a blank (control) group. Log-phase DU145 cells (2×105) were seeded in 24-well plates containing DMEM supplemented with 10% FBS without antibiotics 1 day prior to transfection. Once 70% confluence was reached, 7.5 µl miR-NC, miR-503 mimics and miR-503 inhibitor plasmids (Shanghai GenePharma Co., Ltd, Shanghai, China) and 7.5 µl Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) were added to two individual vials each containing 125 µl serum-free DMEM. The two vials were mixed 5 min later, left for a further 5 min and the mixture was subsequently added to the cells. Following 6 h incubation, the old medium was discarded and fresh DMEM supplemented with 10% FBS was added. The cells were then cultured under normal conditions at 37°C for 48 h prior to use.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
A total of 100 mg tissue was ground into powder using liquid nitrogen prior to the addition of 1 ml TRIzol (Thermo Fisher Scientific, Inc.) for lysis. A total of 2×105 cells were trypsinized and mixed with 1 ml TRIzol for lysis. Total RNA was extracted using the phenol chloroform method (20). The purity of RNA was determined by A260/A280 using ultraviolet spectrophotometry (Nanodrop ND1000, Thermo Fisher Scientific, Inc., Wilmington, DE, USA). cDNA was synthesized by reverse transcription using the Reverse Transcription system (Takara Biotechnology Co., Ltd., Dalian, China) from 1 µg RNA and stored at −20°C.
The expression of miR-503 was determined using a SYBR PrimeScript miRNA RT-PCR kit (Takara Biotechnology Co.). U6 was used as the internal reference. The reaction system (25 µl) contained 12.5 µl SYBR Premix Ex Taq, 1 µl PCR Forward Primer, 1 µl Uni-miR qPCR Primer, 2 µl template and 8.5 µl double distilled water. The primer sequences used were as follows: miR-503, forward, 5′-TGCGGTAGCAGCGGGAACAGTTC-3′ (reverse primer was Uni-miR qPCR Primer that was provided by the kit and its sequence is a commercial secret); and U6, forward 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′. The reaction protocol was as follows: Initial denaturation at 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 20 sec (Bio-Rad IQ5 Realtime PCR; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The 2−ΔΔCq method was used to calculate the relative expression of miR-503 against U6 (21). Each sample was tested in triplicate.
A SYBR Green RT-qPCR kit (Kapa Biosystems, Inc., Wilmington, MA, USA) was used according to the manufacturer's protocol to detect the mRNA expression of TPD52L2 in prostate tissues and DU145 cells. GADPH was used as an internal reference. The reaction system (20 µl) was composed of 10 µl SYBR EX Taq-Mix, 0.5 µl upstream primer, 0.5 µl downstream primer, 1 µl cDNA and 8 µl double diluted water. The primer sequences were as follows: TPD52L2 mRNA, forward, 5′-TTCACAGGCAGGACAGAAGA-3′ and reverse, 5′-TTGAAGGTCGCAGAGTTCCT-3′; GADPH, forward, 5′-AAGGTGAAGGTCGGATCA-3′ and reverse, 5′-GGAAGATGGTGATGGGATTT-3′. The PCR thermocycling conditions were as follows: Initial denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C for 1 min, annealing at 60°C for 40 sec and elongation at 72°C for 30 sec and final elongation at 72°C for 1 min (iQ5; Bio-Rad Laboratories, Inc.). The 2−ΔΔCq method was used to calculate the relative expression of TPD52L2 mRNA against GADPH. Each sample was tested in triplicate.
Western blot analysis
A total of 50 mg tissue was ground using liquid nitrogen. DU145 cells were trypsinized and collected prior to being mixed with 100 µl precooled radioimmunoprecipitation assay lysis buffer (600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM sodium chloride, 0.1% sodium dodecyl sulfate, 1% TritonX-100, 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) for 50 min on ice. The mixture was centrifuged at 12,000 × g at 4°C for 5 min. The supernatant was used to determine protein concentration using a bicinchoninic acid protein concentration determination kit [RTP7102, Real-Times (Beijing) Biotechnology Co., Ltd., Beijing, China]. Protein samples (50 µg) were mixed with 2× sodium dodecyl sulfate loading buffer prior to denaturation in a boiling water bath for 5 min. Subsequently, samples (10 µl) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 100 V. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (300 mA, 1.5 h) and blocked with 5 g/l skimmed milk at room temperature for 1 h. Membranes were incubated with rabbit anti-human TPD52L2 polyclonal primary antibody (1:2,000; catalogue no. ab194938) and rabbit anti-human GAPDH primary antibody (1:2,000; catalogue no. ab37168; both Abcam Cambridge, UK) at 4°C overnight. Following three extensive washes with phosphate-buffered saline and Tween 20 for 15 min, membranes were incubated with polyclonal goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:1,000; catalogue no. ab6721; Abcam) for 1 h at room temperature prior to three washes with phosphate-buffered saline and Tween 20 for 15 min. Membranes were developed using an enhanced chemiluminescence detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for imaging. Image lab v3.0 software (Bio-Rad Laboratories, Inc.) was used to analyze imaging signals. The relative content of TPD52L2 protein was expressed as the ratio of TPD52L2/GAPDH.
Transwell assay
Transwell chambers (Corning Inc., Corning, NY, USA) were used to evaluate the migration ability of DU145 cells. Transfected cells were trypsinized and re-suspended to a density of 5×105 cells/ml using DMEM containing 0.1% bovine serum albumin (Sigma-Aldrich; Merck KGaA). The cell suspension (200 µl; 5×105 cells) was added into the migration chamber (Hyclone, GE Healthcare Life Sciences; Logan, UT, USA). A total of 750 µl DMEM supplemented with 20% fetal bovine serum was added to the lower chamber. After 4 h, cells in the migration chamber were wiped using a cotton swab. Cells that had migrated to the other side of the chamber were fixed with 100% methanol at room temperature for 10 min. Following staining at room temperature with 0.1% crystal violet for 15 min, the number of cells was counted under a microscope (DM1000; Leica Microsystems GmbH, Wetzlar, Germany).
MTT assay
Following transfection, DU145 cells were seeded into 96-well plates at a density of 3,000 cells/well. Each experiment was conducted in triplicate. At 24, 48, and 72 h following transfection, 20 µl MTT (5 g/l; JRDUN Biotechnology, Shanghai, China) was added to each well. Following incubation at 37°C for 4 h dimethyl sulfoxide was used to dissolve purple formazan. The absorbance of each well was measured at 492 nm with a microplate reader and cell proliferation curves were subsequently plotted.
Flat plate colony formation test
DU145 cells from the DU145+miR-503 mimic, DU145+miR-503 inhibitor, NC and control groups were trypsinized and washed twice with phosphate-buffered saline. The number of cells was counted following staining with trypan blue at room temperature and the cells were prepared into a suspension with a density of 0.5×103 cells/ml. The cell suspension (2 ml) was inoculated into 6-well plates, followed by incubation under normal conditions at 37°C. Half the medium was replenished on day 5. The medium was discarded on day 14 and cells were washed once with phosphate-buffered saline. Cells were fixed with methanol at room temperature for 10 min and stained with crystal violet at room temperature for 5 min. Following extensive washing with phosphate-buffered saline, the cells were observed under a microscope (DM1000; Leica Microsystems GmbH) and five fields were selected for colony counting. The colony formation rate was then calculated using the following equation: colony formation rate = (number of clones) / (number of seeded cells) ×100% (22).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Measurement data were expressed as mean ± standard deviation and data from two groups were compared using a Student's t-test. P<0.05 indicated a statistically significant difference.
Results
TPD52L2 expression is elevated in prostate cancer tissues
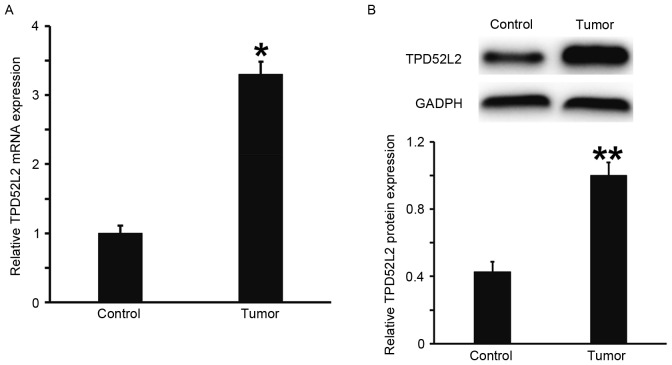
RT-qPCR and western blot analysis were performed to measure the expression of TPD52L2 mRNA and protein in prostate tumor tissues. The results demonstrated that the levels of TPD52L2 mRNA and protein in prostate cancer tissues were significantly increased compared with those from tumor-adjacent normal tissues (P<0.05; Fig. 1A and B). This suggests that the expression of TPD52L2 is elevated in prostate cancer tissues.
Figure 1.
Expression of TPD52L2 (A) mRNA and (B) protein in prostate cancer tissues and tumor-adjacent normal tissues. Reverse transcription-quantitative polymerase chain reaction was used to determine the expression of TPD52L2 mRNA and western blot analysis was used to measure TPD52L2 protein expression. *P<0.05 and **P<0.01 vs. control. TPD52L2, tumor protein D52 like 2.
Expression of miR-503 in prostate cancer tissues is reduced, which may be due to the direct interaction between miR-503 and TPD52L2
TargetScan was used to predict the direct interaction between miR-503 and TPD52L2, and RT-qPCR was performed to measure the expression of miR-503 in prostate cancer tissues. TargetScan identified the 3′-untranslated region (3′UTR) of TPD52L2 as a direct target of miR-503 (Fig. 2A). RT-qPCR data determined that miR-503 levels in prostate cancer tissues were significantly decreased compared with those in tumor-adjacent normal tissues (P<0.01; Fig. 2B). These results indicate that the expression of miR-503 in prostate cancer tissues was decreased, which may be due to the direct interaction of miR-503 with TPD52L2.
Figure 2.
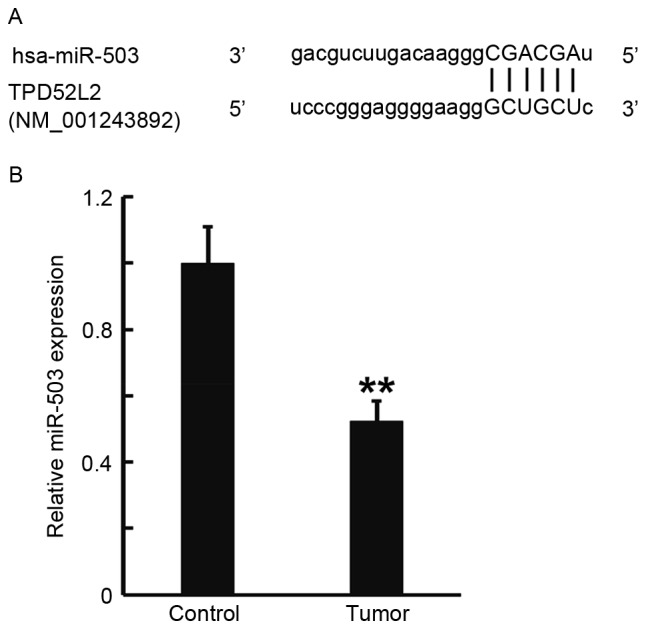
Expression of miR-503 in prostate cancer tissues and tumor-adjacent normal tissues. (A) Bioinformatics prediction of direct interactions between miR-503 and TPD52L2 using TargetScan. (B) Expression of miR-503 as determined by reverse transcription quantitative polymerase chain reaction. **P<0.01 vs. control. miR-503, microRNA-503; TPD52L2, tumor protein D52 like 2.
Overexpression of miR-503 inhibits the transcription and translation of TPD52L2
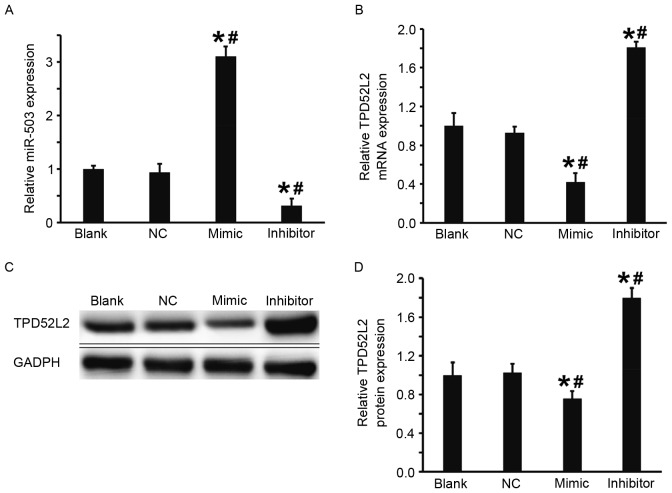
The DU145 cells were transfected with miR-503 mimic or miR-503 inhibitor to examine the regulation of TPD52L2 gene expression by miR-503 in DU145 cells. Compared with the blank and NC groups, the expression of miR-503 in DU145 cells transfected with miR-503 mimic was significantly increased and in DU145 cells transfected with miR-503 inhibitor it was significantly decreased (both P<0.05; Fig. 3A). Furthermore, the results from RT-qPCR and western blot analysis demonstrated that in comparison with the blank and NC groups, levels of TPD52L2 mRNA and protein in the DU145+miR-503 mimic group were significantly decreased and in the DU145+miR-503 inhibitor group they were significantly increased (all P<0.05; Fig. 3B-D). These results suggest that overexpression of miR-503 inhibits the transcription and translation of TPD52L2.
Figure 3.
Effect of miR-503 on the expression of TPD52L2 in DU145 cells. (A) Expression of miR-503 in the DU145 cells of blank, NC, mimic and inhibitor groups. (B) Relative expression of TPD52L2 mRNA in DU145 cells of the blank, NC, mimic and inhibitor groups. (C-D) Relative expression of TPD52L2 protein in DU145 cells of blank, NC, mimic and inhibitor groups. Reverse transcription-quantitative polymerase chain reaction was used to determine the expression of miR-503 and TPD52L2 mRNA and western blot analysis was used to measure TPD52L2 protein expression. *P<0.05 vs. blank group; #P<0.05 vs. NC group. miR-503, microRNA-503; NC, negative control; TPD52L2, tumor protein D52 like 2.
Overexpression of miR-503 suppresses the migration ability of DU145 cells
A Transwell assay was performed to investigate the effect of miR-503 on the migration of DU145 cells. The results demonstrated that, compared with the blank and NC groups, the number of migrated cells in the DU145+miR-503 mimic group was significantly decreased and in the DU145+miR-503 inhibitor group it was significantly increased (all P<0.05; Fig. 4). These results indicate that the overexpression of miR-503 suppresses the migration ability of DU145 cells.
Figure 4.
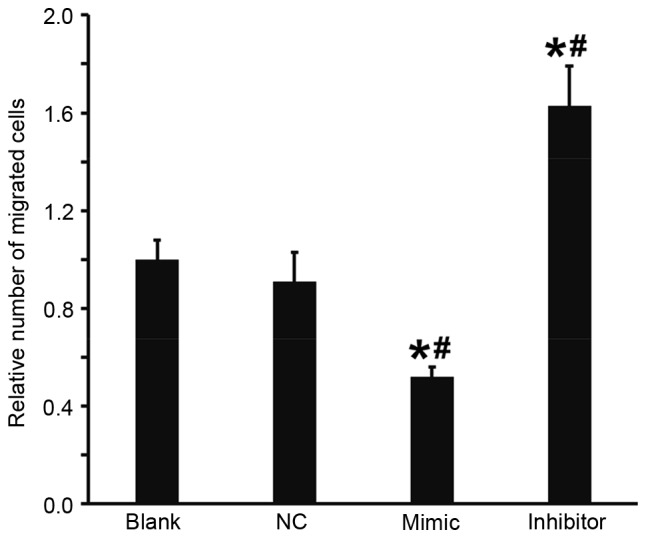
Effect of microRNA-503 on the migration ability of DU145 cells. Transwell assay was used to determine the migration ability of the cells. *P<0.05 vs. blank group; #P<0.05 vs. NC group. NC, negative control.
miR-503 inhibits the proliferation of DU145 cells
An MTT assay was performed to determine the effect of miR-503 on the proliferation of DU145 cells. Compared with the blank and NC groups, the absorbance of cells in the DU145+miR-503 mimic group was significantly decreased and in the DU145+miR-503 inhibitor group it was significantly increased at 48 and 72 h (all P<0.05; Fig. 5). This suggests that miR-503 inhibits the proliferation of DU145 cells.
Figure 5.
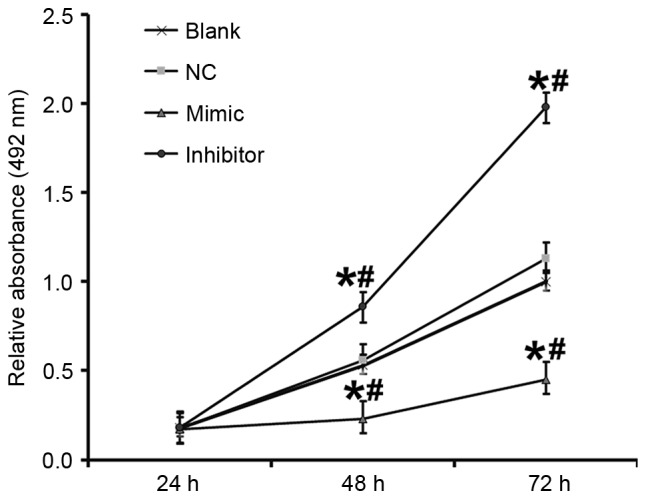
Proliferation of DU145 cells at 24, 48 and 72 h following transfection. An MTT assay was used to determine the proliferation of cells. The absorbance of each well was measured at 492 nm using a microplate reader and cell proliferation curves were plotted. *P<0.05 vs. blank group; #P<0.05 vs. NC group. NC, negative control.
Overexpression of miR-503 reduces the colony formation ability of DU145 cells
A flat plate colony formation test was used to determine how miR-503 affects the colony formation ability of DU145 cells. Colony counting demonstrated that compared with the blank and NC groups, the colony formation rate of DU145 cells in the DU145+miR-503 mimic group was significantly decreased and in the DU145+miR-503 inhibitor group it was significantly increased (both P<0.05; Fig. 6). These results indicate that the overexpression of miR-503 reduces the colony formation ability of DU145 cells.
Figure 6.
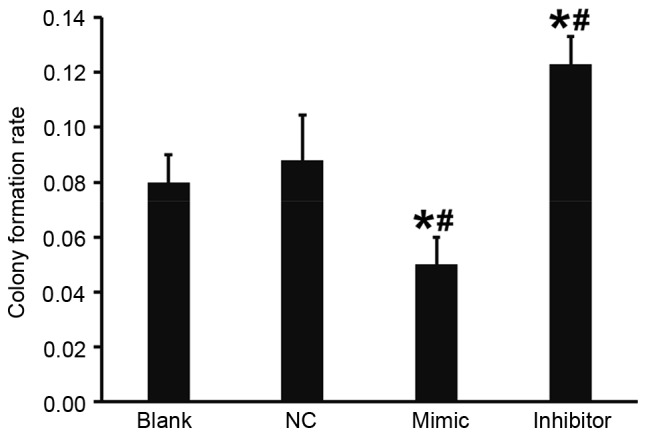
Colony formation ability of DU145 cells. Colony formation rates of DU145 cells in blank, negative control, mimic and inhibitor groups were determined using the flat plate colony formation test. *P<0.05 vs. blank group; #P<0.05 vs. NC group. NC, negative control.
Discussion
miRNA acts as an oncogene or tumor-suppressor gene by binding to the 3′-UTR seeding sequence of mRNA (23). It has been demonstrated that miR-503 is downregulated in hepatic tissues and cells and inhibits tumor angiogenesis by acting on fibroblast growth factor 2 and vascular endothelial growth factor A (24), suggesting that it is an important tumor-suppressor factor. However, the mechanism of action of miR-503 in prostate cancer remains unclear.
The results of the present study demonstrate that the expression of miR-503 in prostate cancer tissues is significantly lower than in tumor-adjacent normal tissues and bioinformatic analysis indicated that TPD52L2 is a potential target gene of miR-503. Ummanni et al (25) demonstrated that TPD52 promotes αVβ3 integrin-mediated cell migration in prostate cancer by activating the phosphatidylinositol 3-kinase/Akt signaling pathway and the mitochondrial apoptotic response as an upstream response element. The present study demonstrated that the expression of TPD52L2 mRNA and protein in prostate cancer tissues was higher than in normal tissues, suggesting that miR-503 may be associated with TPD52L2. Transfection with miR-503 mimic or miR-503 inhibitor induces the overexpression or inhibition of miR-503 expression in DU145 cells, respectively. The overexpression of miR-503 in DU145 cells inhibits the expression of TPD52L2, and by contrast, inhibition of miR-503 elevates the expression of TPD52L2. The present study on cellular function has demonstrated that DU145 cells overexpressing miR-503 exhibit decreased migration, proliferation and colony formation, whereas cells with reduced miR-503 expression have improved migration, proliferation and colony formation functions. Previous studies have identified that miR-503 is abnormally expressed in retinal glioblastoma, adenoma, breast cancer, non-small cell lung cancer and hepatic cancer. Furthermore, it has been demonstrated that miR-503 targets phosphoinositide-3-kinase, p85 or inhibitor of nuclear factor κB kinase subunit β, regulates the Rho guanine nucleotide exchange factor 19, DDHD domain containing 2 and F-box and WD repeat domain containing 7 genes and initiates apoptosis, thus promoting the growth and differentiation of tumors and inhibiting the invasion and metastasis of tumors (18,26–31). This indicates that miR-503 serves important roles in different types of cancer.
In conclusion, the present study demonstrated that miR-503 expression in prostate cancer cells is negatively associated with the migration ability of cells and suggested that miR-503 may serve important roles in the onset and development of prostate cancer by targeting TPD52L2. Therefore, the current study provides novel insights into the mechanisms of prostate cancer.
Acknowledgements
The present study was supported by the People's Hospital of Rizhao. The authors also wish to thank Professor Shuguang Yang and Professor Zhaojun Ding for their valuable help.
References
- 1.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 2.Wong YC, Wang YZ. Growth factors and epithelial-stromal interactions in prostate cancer development. Int Rev Cytol. 2000;199:65–116. doi: 10.1016/S0074-7696(00)99002-8. [DOI] [PubMed] [Google Scholar]
- 3.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaney JM, Wang S, Funda S, Long J, Taghipour DJ, Tbaishat R, Furbert-Harris P, Ittmann M, Kwabi-Addo B. Identification of novel DNA-methylated genes that correlate with human prostate cancer and high-grade prostatic intraepithelial neoplasia. Prostate Cancer Prostatic Dis. 2013;16:292–300. doi: 10.1038/pcan.2013.21. [DOI] [PubMed] [Google Scholar]
- 5.Marcelli M, Ittmann M, Mariani S, Sutherland R, Nigam R, Murthy L, Zhao Y, DiConcini D, Puxeddu E, Esen A, et al. Androgen receptor mutations in prostate cancer. Cancer Res. 2000;60:944–949. [PubMed] [Google Scholar]
- 6.Boutros R, Fanayan S, Shehata M, Byrne JA. The tumor protein D52 family: Many pieces, many puzzles. Biochem Biophys Res Commun. 2004;325:1115–1121. doi: 10.1016/j.bbrc.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DD, Martin CL, Weng N, Byrne JA, Groblewski GE. Tumor protein D52 expression and Ca2+-dependent phosphorylation modulates lysosomal membrane protein trafficking to the plasma membrane. Am J Physiol Cell Physiol. 2010;298:C725–C739. doi: 10.1152/ajpcell.00455.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balleine RL, Fejzo MS, Sathasivam P, Basset P, Clarke CL, Byrne JA. The hD52 (TPD52) gene is a candidate target gene for events resulting in increased 8q21 copy number in human breast carcinoma. Genes Chromosomes Cancer. 2000;29:48–57. doi: 10.1002/1098-2264(2000)9999:9999<::AID-GCC1005>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Chen CL, Pan QZ, Wu YY, Zhao JJ, Jiang SS, Chao J, Zhang XF, Zhang HX, Zhou ZQ, et al. Decreased TPD52 expression is associated with poor prognosis in primary hepatocellular carcinoma. Oncotarget. 2016;7:6323–6334. doi: 10.18632/oncotarget.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Sun J, Zhao Y, Guo W, Lv K, Zhang Q. Lentivirus-mediated knockdown of tumor protein D52-like 2 inhibits glioma cell proliferation. Cell Mol Biol (Noisy-le-grand) 2014;60:39–44. [PubMed] [Google Scholar]
- 11.Pan ZY, Yang Y, Pan H, Zhang J, Liu H, Yang Y, Huang G, Yin L, Huang J, Zhou WP. Lentivirus-mediated TPD52L2 depletion inhibits the proliferation of liver cancer cells in vitro. Int J Clin Exp Med. 2015;8:2334–2341. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Almeida MI, Reis RM, Calin GA. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat Res. 2011;717:1–8. doi: 10.1016/j.mrfmmm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 14.Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 15.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao JJ, Yang J, Lin J, Yao N, Zhu Y, Zheng J, Xu J, Cheng JQ, Lin JY, Ma X. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis. Childs Nerv Syst. 2009;25:13–20. doi: 10.1007/s00381-008-0701-x. [DOI] [PubMed] [Google Scholar]
- 17.Watahiki A, Wang Y, Morris J, Dennis K, O'Dwyer HM, Gleave M, Gout PW, Wang Y. MicroRNAs associated with metastatic prostate cancer. PLoS One. 2011;6:e24950. doi: 10.1371/journal.pone.0024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu T, Zhou L, Wang T, Xu J, Wang J, Chen W, Zhou X, Huang Z, Zhu W, Shu Y, Liu P. miR-503 regulates the resistance of non-small cell lung cancer cells to cisplatin by targeting Bcl-2. Int J Mol Med. 2013;32:593–598. doi: 10.3892/ijmm.2013.1439. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. ISUP Grading Committee: The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Ma H, Sun J. microRNA-34a/c function as tumor suppressors in Hep-2 laryngeal carcinoma cells and may reduce GALNT7 expression. Mol Med Rep. 2014;9:1293–1298. doi: 10.3892/mmr.2014.1929. [DOI] [PubMed] [Google Scholar]
- 23.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 24.Zhou B, Ma R, Si W, Li S, Xu Y, Tu X, Wang Q. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013;333:159–169. doi: 10.1016/j.canlet.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Ummanni R, Teller S, Junker H, Zimmermann U, Venz S, Scharf C, Giebel J, Walther R. Altered expression of tumor protein D52 regulates apoptosis and migration of prostate cancer cells. FEBS J. 2008;275:5703–5713. doi: 10.1111/j.1742-4658.2008.06697.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Tao Y, Peng C, Gu P, Wang W. miR-503 regulates metastatic function through Rho guanine nucleotide exchanger factor 19 in hepatocellular carcinoma. J Surg Res. 2014;188:129–136. doi: 10.1016/j.jss.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Liu L, Zhang Y, Guan H, Wu J, Zhu X, Yuan J, Li M. MiR-503 targets PI3K p85 and IKK-β and suppresses progression of non-small cell lung cancer. Int J Cancer. 2014;135:1531–1542. doi: 10.1002/ijc.28799. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Ge G, Ding Y, Zhou X, Huang Z, Zhu W, Shu Y, Liu P. MiR-503 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R and BCL2. Chin Med J (Engl) 2014;127:2357–2362. [PubMed] [Google Scholar]
- 29.Li L, Sarver AL, Khatri R, Hajeri PB, Kamenev I, French AJ, Thibodeau SN, Steer CJ, Subramanian S. Sequential expression of miR-182 and miR-503 cooperatively targets FBXW7, contributing to the malignant transformation of colon adenoma to adenocarcinoma. J Pathol. 2014;234:488–501. doi: 10.1002/path.4407. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Guo L, Liu J, Liu R, Liu M, Chen J. The reversing and molecular mechanisms of miR-503 on the drug-resistance to cisplatin in A549/DDP cells. Zhongguo Fei Ai Za Zhi. 2014;17:1–7. doi: 10.3779/j.issn.1009-3419.2014.01.01. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polioudakis D, Abell NS, Iyer VR. miR-503 represses human cell proliferation and directly targets the oncogene DDHD2 by non-canonical target pairing. BMC Genomics. 2015;16:40. doi: 10.1186/s12864-015-1279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]