Abstract
Background:
Lichen planus (LP) is a chronic, inflammatory disease that affects the skin, mucous membrane, scalp and nails that frequently involves the oral mucosa. Oxidative stress reflects an imbalance between the production of reactive oxygen species and the biological system's ability to readily detoxify the reactive intermediates or repair the resulting damage. It has been suggested that oxidative stress may play a role in the pathogenesis of LP.
Aim and Objectives:
To evaluate the role of oxidative parameters in the pathogenesis of oral LP, estimate the levels of superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GPx) and uric acid (UA) in saliva of oral LP patients and to compare the levels of SOD, MDA, GP and UA in oral LP patients with healthy controls.
Methodology:
In this cross-sectional study, 1.5 ml of fasting saliva sample was collected using passive drool method from the study group (30 patients diagnosed as having oral LP) and the control group (30 age-matched healthy volunteers). The unstimulated saliva was collected were analyzed by spectrophotometry. Statistical analysis was done to evaluate and compare the values between groups.
Results:
The mean values of SOD and MDA in saliva in the study group showed a significant increase in amount when compared with the control group whereas GPx showed a significant decrease in the study group. UA value showed an insignificant difference in the same comparison.
Interpretation and Conclusion:
Oxidative stress markers as MDA and SOD are elevated, and GPx is decreased in the saliva of oral LP patients.
Keywords: Oral lichen planus, oxidative stress, spectrophotometry
INTRODUCTION
Lichen planus (LP) is a unique, common inflammatory disorder affecting the skin, mucous membrane, nails and hair that frequently affects the oral mucosa. Oral LP (OLP) affects approximately 0.1%–2% of the general adult population.[1] Clinically, OLP can present six different patterns: papule, reticular, plaque, atrophic, erosive and bullous each showing specific characteristics and appearing in either isolated or associated forms.[2,3]
Both antibodies and T-cell-mediated activity have been implicated in the pathogenesis of LP.[1] Studies have reported that oxidative stress may play a role in OLP.[4] Oxidative stress is defined as the imbalance between the production of reactive oxygen species (ROS) and the ability of the biological system to readily detoxify the reactive intermediates or easily repair the resulting damage. This results in the production of free radicals that can damage cell membranes.[5] To defend such damage, the body possesses several antioxidant systems which prevent oxidative stress. Somebody fluids like saliva contain such activity, as saliva is naturally composed of several antioxidants (i.e., uric acid [UA], glutathione and ascorbic acid) and this defensive mechanism is called salivary antioxidant system.[6] Studies have shown that salivary and plasma levels of total antioxidant status in erosive LP patients were lower than those in healthy controls. The inflammatory cellular infiltrates in LP, which consists mainly of CD4+ lymphocytes, is a well-known source of ROS.[7]
Superoxide dismutase (SOD) is considered the first line defense against ROS, converting the superoxide anion (O2−) into H2O2.[8,9] Glutathione peroxidase (GPx) catalyzes the reduction of hydrogen peroxide and lipid hydroperoxides. GPx in combination with catalase and SOD function to protect the cell from damage due to ROS.[9,10] Malondialdehyde (MDA) is used as an indicator of lipid peroxidation.[8,9] UA is the most important antioxidant molecule in saliva.[11]
The present study was undertaken to evaluate the levels of oxidative parameters as SOD, MDA, GPx and UA in saliva of oral LP patients as they serve as early markers of oxidative stress and thus to evaluate the role of these in the pathogenesis of oral LP. SOD and GPx are enzymatic antioxidants; UA is a nonenzymatic antioxidant and MDA is an oxidant.
The objectives were to estimate the levels of SOD, MDA, GP and UA in saliva of oral LP patients and to compare their levels with that of healthy controls.
METHODOLOGY
In this cross-sectional study, Group I (study group) consisted of 30 healthy volunteers, and Group II (control group) consisted of 30 patients diagnosed as having oral LP [Figures 1 and 2]. The members of the study group were selected from the patients attending the OP department of our institution from September 2013 to August 2014. Patients were selected by simple random sampling according to inclusion and exclusion criteria. Patients diagnosed as having oral LP, who were willing to participate in the study, were included in this study. Patients with any other systemic illnesses and oral diseases as aphthous ulcers, gingivitis; those on systemic or topical medication, supplementary vitamins for the past 2 months were excluded from the study. Patients with a history of trauma or surgery, with a history of alcoholism, chewing habit and smoking; taking drugs for LP for the past 2 months were also excluded, to avoid bias from the effects of the drugs on salivary contents.
Figure 1.
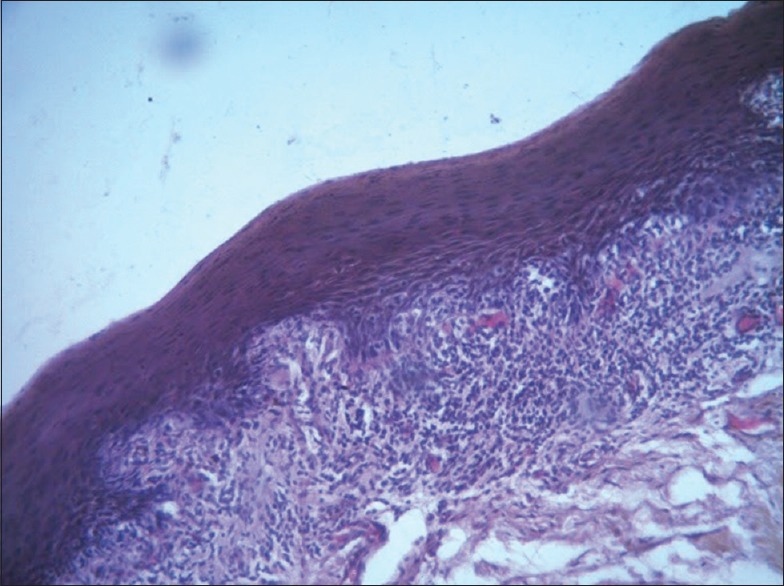
Photomicrograph of lichen planus showing epithelium with elongated rete ridges and dense subepithelial inflammation (H & E, x10)
Figure 2.
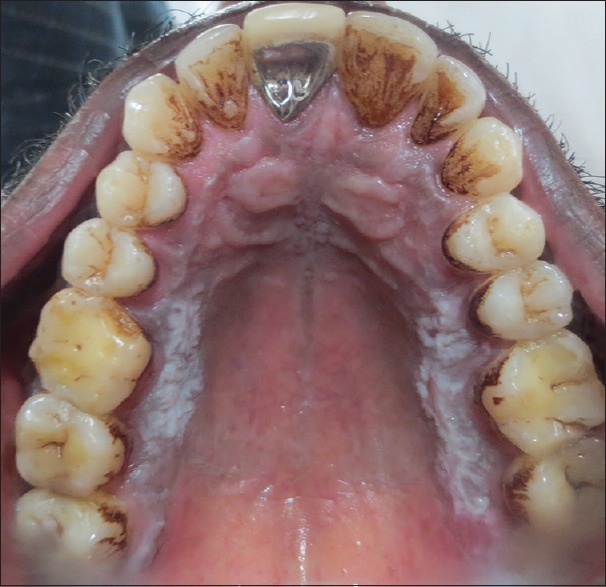
Clinical photograph showing lichen planus on palatal mucosa adjacent to molar teeth bilaterally
The study was commenced on obtaining clearance from Institutional Ethics Committee. Before initiating the study, a written informed consent in accordance with ethical codes adopted by National Committee for Medical Research Ethics was completed by all participants.
A volume of 1.5 ml of fasting saliva sample was collected from the Groups I and II. Saliva collection was done using passive drool method (Wainwright and Kerr).[12]
The unstimulated saliva was collected from patient between 8.00 am and 10.00 am. The patients were asked to rinse their mouth thoroughly with water 10 min before saliva collection, and they were asked to spit out or swallow saliva already present in the mouth. After the individuals were comfortably seated and after a few minutes of relaxation, they were trained to avoid swallowing saliva and asked to lean forward and drool all the saliva they produced into a vial using a custom-made saliva collecting funnel over a period of 5–10 min sufficient amount of saliva was collected. Once collected, saliva containing vials were placed in an ice carrier box and immediately transferred to the laboratory for biochemical analysis. Saliva samples were stored at −20°C until analysis. In the laboratory, analysis was done immediately.
Saliva samples were centrifuged at 3000 rpm for 10 min at 4°c. The supernatants were collected and used for enzyme studies.
Estimation of superoxide dismutase activity
Fifty microliters (50 μl) of saliva samples were added to a test tube containing 3 ml of the reaction mixture (50 mM potassium phosphate buffer [7.8], 45 μM methionine, 5.3 mM riboflavin, 84 μM nitroblue tetrazolium [NBT] and 20 μM potassium ferric cyanide). The tubes were incubated in 25°C for 10 min and read on spectrophotometer at 600 nm (Mishra and Fridovish, 1977).[13]
SOD activity was analyzed by the reduction of NBT by superoxide, which formed formazan and detected spectrometrically at 560 nm using ultraviolet spectrophotometer and expressed in terms of U/ml.
Estimation of malondialdehyde
Fifty microliters (50 μl) of saliva samples were mixed with 500 μl of 70% alcohol and 1 ml of 1% TBA. Then, all the tubes were kept in boiling water bath for 20 min. After cooling to room temperature added 50 μl of acetone to all the test tubes and read absorbance at 535 nm in spectrophotometer. The MDA values were compared with a standard MDA graph.[14]
The MDA levels were estimated by thiobarbituric UA TBA reaction using trichloroacetic acid TCA. The end products of lipid peroxidation particularly MDA react with thiobarbituric UA under acidic condition and heating to give a pink color that could be measured spectrophotometrically at 532 nm.
Estimation of glutathione peroxidase
Fifty microliters of saliva samples were added to a test tube containing 3 ml of reaction mixture (1 mM of β-NADPH+1 mM sodium azide solution, 200 mM reduced glutathione). Mixed by inversion and equilibrated to 25°C and monitored the absorbance at 340 nm until constant. The tube containing 3 ml reaction mixture and 50 μl of phosphate buffer (pH 7) was taken as blank. 50 μl of 0.042% of hydrogen peroxide was added to these tubes. Immediately mixed by inversion and recorded the decrease in absorbance at 340 nm for approximately 5 min.
The enzyme GP catalyzes the oxidation of reduced GSH to oxidized form, which reacts with NADPH and gets converted to oxidized form of NADP and two molecules of reduced glutathione and is measured spectrophotometrically at 340 nm.
Estimation of uric acid
UA concentration was measured in the saliva of the participants, using a kit supplied by Erba diagnostics named UA DES. To 20 μl sample, 1 ml working reagent was added. Working reagent was prepared by making the vial and Aqua-4 to attain the room temperature (15°C–30°C) and then adding Aqua-4 (100 ml) to the contents of each vial and mixing gently to completely dissolve. Working reagent and sample were mixed and incubated for 5 min at 37°C. At the same time, blank and standard solution was taken. The absorbance of the standard and each test are read at 546 nm against reagent blank.
UA is transformed by uricase into allantoin and hydrogen peroxide which, under the catalytic influence of peroxidase, oxidizes the chromogen (4-aminophenazone/N-ethyl-methylanilin propane-sulfonate sodium) to form a red compound the intensity of color of which is proportional to the amount of UA present in the sample and which is read at a wavelength of 546 nm.
RESULTS
The present study was done to evaluate the role of oxidative parameters in the pathogenesis of oral LP. The levels of SOD, MDA, GPx and UA were measured in saliva of oral LP patients and compared among two groups
Group I (study group) – Oral LP patients
Group II (controls) – Normal individuals
Both groups were age and sex matched.
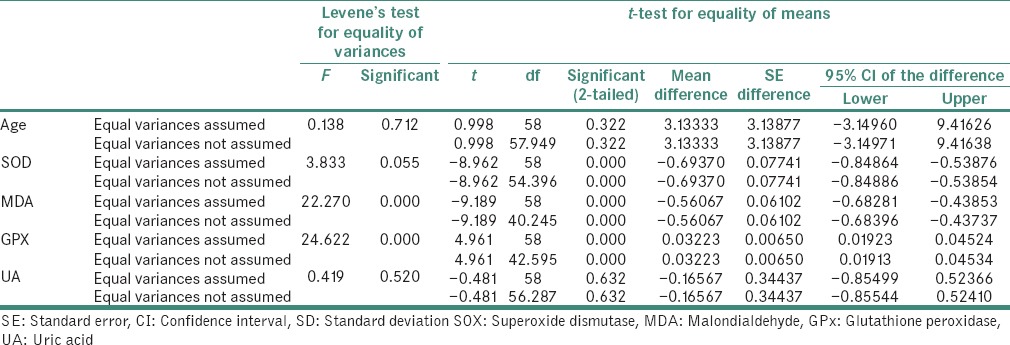
The data analysis was done using Statistical Package for Social Sciences (IBM SPSS version 20). Independent sample t-test applied to find the statistical significant between the groups. (P < 0.05) considered statistically significant at 95% confidence interval. The data expressed in mean ± standard deviation (SD).
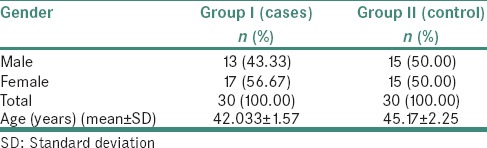
In the present study, out of 30 cases, 13 were males (43.33%) and 17 were females (56.67%). Out of the 30 controls, 15 were males (50%) and 15 were females (50%) [Table 1 and Graph 1].
Table 1.
Distribution of patients according to gender in the study and control groups
Graph 1.
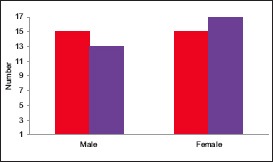
Distribution of patients according to gender in the study and control groups (cases; controls)
The mean age of cases was 42.033 ± 1.57 and that of controls was 45.17 ± 2.25 [Table 1].
The different sites include buccal mucosa, alveolar mucosa, tongue, gingiva, palate, lips with most cases involving multiple sites and a bilaterally symmetrical distribution. Different clinical variants as reticular, erosive, atrophic and plaque-like were included.
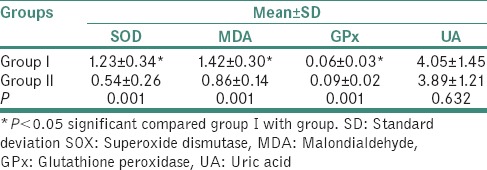
The mean values of SOD, MDA, GPx and UA in the study group and the control group are tabulated tabulated in Table 2 and plotted in Graph 2.
Table 2.
Comparison of salivary antioxidant levels between study and control groups
Graph 2.
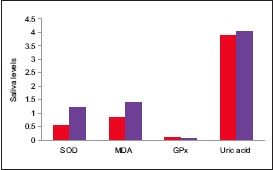
Comparison of mean salivary antioxidant levels between study and control groups
Comparison of salivary antioxidant levels between study and control groups
The mean value of SOD in the study group was 1.23 ± 0.34 and in the control group was 0.54 ± 0.26., the difference being statistically significant (P = 0.001). The mean value of MDA in the study group was 1.42 ± 0.30 and in the control group was 0.86 ± 0.14, the difference being statistically significant (P = 0.001). The mean value of GPx in the study group was 0.06 ± 0.03 and in the control group was 0.09 ± 0.02, the difference being statistically significant (P = 0.001). The mean value of UA in the study group was 4.05 ± 1.45 and in the control group was 3.89 ± 1.21, the difference being statistically insignificant (P = 0.632) [Table 3].
Table 3.
Independent Sample t Test
DISCUSSION
Saliva has been used in the past few decades as a new diagnostic fluid.[15] The use of saliva as a diagnostic tool presents many advantages: it is easy to collect, by a noninvasive technique; no special equipment is needed for collection. Collection of saliva is associated with fewer compliance problems compared with blood collection, and salivary levels correlate well with serum levels.[16,17] Hence, this study was undertaken in saliva of oral LP patients, which is known to be a potentially malignant condition. Anshumalee et al. and Sezer et al. reported that oxidative stress and ROS may be involved in the pathogenesis of the LP.[4,18]
In a study by Sezer et al. in 2007 SOD activity was determined in 40 oral LP patients. Serum SOD levels (18.19 ± 3.71 U/mL) in patients with LP were also higher than in healthy controls (P = 0.002) which was similar to the present study on saliva.[18] In a study by Hassan et al. in 2013, SOD activity was estimated, and the mean value of plasma SOD in cases was 5.32 ± 0.57 U/ml, while in controls, the mean value was 4.07 ± 0.99 U/ml. This difference was statistically significant (P < 0.0001).[19] These results were also consistent with the present study.
Aly and Shahin in 2010 included 45 Egyptian LP patients and 45 healthy volunteers as controls and conducted a study in which serum levels of SOD were higher in LP patients with mean ± SD of 17.33 ± 2.05 when compared to controls (P = 0.009) leading to an imbalance in the antioxidant defense system. This study showed a positive correlation between nitric oxide NO, MDA and SOD and the duration of LP. No relation between SOD and the clinical types of LP was noted.[20]
Serum SOD levels were found significantly lower in oral LP patients in a study by Jingyan et al., 2001 in 42 OLP patients before the treatment than those in healthy controls (P = 0.001), while after treatment, the SOD levels increased and LPO levels decreased significantly in OLP patients, and no significant difference were found as compared with healthy controls (P = 0.05).[21]
In general, different studies reveal a positive correlation between the serum and salivary levels of antioxidants.[16,22,23,24]
Malondialdehyde
MDA is the principal and most studied end product of polyunsaturated fatty acid peroxidation. It has been considered a good marker of free radical-mediated damage and oxidative stress. Thiobarbituric UA Reactive Substances Assay TBARS is the most commonly employed method of MDA estimation.[25]
The present study revealed that salivary MDA levels were significantly higher in OLP than in controls, which were consistent with previous studies by Agha-Hosseini et al.[22] and Ergun et al.[5] According to studies by Sezer et al.,[18] Rai et al.,[26] Aly and Shahin,[20] Upadhyay et al.[27] and Scrobota et al.[28] serum MDA levels were found significantly higher in OLP than in controls. In a study by Abbas et al. in 2014 showed that the mean level of salivary MDA in patients with OLP (0.972 ± 0.433 μmol/l) was significantly higher (P < 0.05) than that of control group (0.732 ± 0.358 μmol/l).[29]
Agha-Hosseini et al. in 2009 in their study in thirty patients with OLP stated that the mean level of unstimulated whole saliva MDA in patients with OLP was significantly higher than that of the control group (t = 2.34, P < 0.05).[22,24] Shirzad et al. in 2014 found levels of salivary MDA as 0.49 ± 0.30 μM; remarkably higher in oral LP patients compared to the control group (0.15 ± 0.11 μM) (P < 0.0001).[30]
Glutathione peroxidase
GP is a well-known enzyme that forms the first line of defense against oxidative stress, which in turn requires glutathione as a cofactor.[31,32]
During oxidative stress the ratio of reduced glutathione GSH/oxidized glutathione GSSG has been changed, on the one hand, GSH is rapidly consumed by GPx, on the other hand, oxidative glutathione generated by glutathione reductase should be provided as reduced glutathione to the site. This mechanism may not occur fast enough in the existence of high concentration of H2O2 for a long time and because of enough unprovided GSH as well as due to oxidative damages of free radicals existing in the site on GSH and GPx, the activity of GPx may have decreased. The reason why the GPx activity in saliva was low in our study was due to, we believe, possible high concentration of H2O2 in lesion area.
Hassan et al. in 2013 conducted a study in which plasma GPx was assayed according to the method of Beutler in 1989. Plasma GPx levels were lower in patients as compared to controls, which is in accordance with the present study in saliva. Mean value of plasma GPx in patients was 47.32 U/L ± 2.46, while in controls, its mean value was 49.0 U/L ± 3.26, the difference being statistically significant (P < 0.001).[19] A study by Barikbin et al. in 2011 in 30 patients of LP revealed a significant positive correlation between selenium and GPx in serum of both patients and controls.[33]
Scrobota et al. in 2010 found the median level of glutathione GSH higher in LP patient's serum as compared to control group.[30] Scrobota et al. in 2011, in their study in nine biopsied oral tissue specimens of LP patients analyzed GSH. GSH medium level was found significantly decreased in patients tissue compared to controls.[34] This may be due to the rate of GSH turnover augmented as a defense mechanism against oxidative stress.
Uric acid
UA is an important salivary biomarker with clinical importance in monitoring the oxidative stress.[11,35] Studies have shown that UA was significantly decreased in OLP patients group, as compared with controls.[11] This may be due to possible high concentration of hydroxyl group in lesion area.
But salivary UA was found to be significantly increased in most pathological conditions such as cerebrovascular accident, diabetes, cardiovascular disorders and recurrent aphthous stomatitis, as is likely to be directly affected by systemic oxidative stress.[36,37,38,39]
The little increase of UA in our study may represent a compensatory antioxidant defense system to counteract oxidative stress.
Battino et al. in 2008 showed a significant decrease of saliva (P < 0.005) UA and an increase in serum gamma-glutamyl transferase GGT (P < 0.01), as well as in the total antioxidant capacity of saliva, in patient group with respect to the control one.[6] According to a study by Miricescu et al. in 2011 in 20 oral LP patients, salivary UA was found to be 1.5–2 mg/dl in them, whereas the value was above 3 mg/dl in controls.[11]
CONCLUSION
Oral LP is a chronic mucocutaneous disease with a high prevalence rate. Oxidative stress markers such as MDA and SOD are elevated and GPx is decreased in the saliva of oral LP patients.
Saliva testing has been found as a noninvasive alternative to serum testing. The aim of salivary analysis is mainly for screening which may be helpful in the future. In the present study, SOD, MDA, GPx and UA were evaluated in the saliva of oral LP patients. The mean values of SOD and MDA in the study group showed a significant increase when compared with the control group, whereas GPx showed a significant decrease in the study group. UA values showed an insignificant difference in the same comparison. Salivary antioxidant levels show a significant difference in response to oxidative stress in oral LP patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rajendran R. Diseases of the skin. In: Rajendran R, Sivapathasundaram S, editors. Shafer's Textbook of Oral Pathology. New Delhi: Reed Elsevier India Private Limited; 2012. pp. 805–40. [Google Scholar]
- 2.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 3rd ed. Philadelphia: W.B. Saunders Company; 2009. [Google Scholar]
- 3.Mollaoglu N. Oral lichen planus: A review. Br J Oral Maxillofac Surg. 2000;38:370–7. doi: 10.1054/bjom.2000.0335. [DOI] [PubMed] [Google Scholar]
- 4.Anshumalee N, Shashikanth MC, Sharma S. Oxidative stress and oral lichen planus: A possible association? Cusp. 2007;4:31–4. [Google Scholar]
- 5.Ergun S, Troşala SC, Warnakulasuriya S, Özel S, Önal AE, Ofluoǧlu D, et al. Evaluation of oxidative stress and antioxidant profile in patients with oral lichen planus. J Oral Pathol Med. 2011;40:286–93. doi: 10.1111/j.1600-0714.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- 6.Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. The antioxidant capacity of saliva. J Clin Periodontol. 2002;29:189–94. doi: 10.1034/j.1600-051x.2002.290301x.x. [DOI] [PubMed] [Google Scholar]
- 7.Azizi A, Farshchi F. Comparison of salivary and plasma antioxidant levels in lichen planus patients and healthy subjects. J Oral Pathol Med. 2012;41:524–6. doi: 10.1111/j.1600-0714.2012.01138.x. [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: Oxidative damage and pathogenesis. Curr Sci. 1999;77:658–66. [Google Scholar]
- 9.Nagler RM, Klein I, Zarzhevsky N, Drigues N, Reznick AZ. Characterization of the differentiated antioxidant profile of human saliva. Free Radic Biol Med. 2002;32:268–77. doi: 10.1016/s0891-5849(01)00806-1. [DOI] [PubMed] [Google Scholar]
- 10.Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–7. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 11.Miricescu D, Greabu M, Tan A, Didilescu A, Radulescu R. The antioxidant potential of saliva: Clinical significance in oral diseases. Ther Pharmacol Clin Toxicol. 2011;15:139–43. [Google Scholar]
- 12.Stephen KW, Speirs CF. Methods for collecting individual components of mixed saliva: The relevance to clinical pharmacology. Br J Clin Pharmacol. 1976;3:315–9. doi: 10.1111/j.1365-2125.1976.tb00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra HP, Fridovich I. Superoxide dismutase: “positive” spectrophotometric assays. Anal Biochem. 1977;79:553–60. doi: 10.1016/0003-2697(77)90429-8. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Spielmann N, Wong DT. Saliva: Diagnostics and therapeutic perspectives. Oral Dis. 2011;17:345–54. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greabu M, Battino M, Mohora M, Totan A, Didilescu A, Spinu T, et al. Saliva – A diagnostic window to the body, both in health and in disease. J Med Life. 2009;2:124–32. [PMC free article] [PubMed] [Google Scholar]
- 17.Singh M, Sau S, Bhat M, Pragna Y, Bommi D. Non-invasive diagnostic tool for pathological conditions: Salivary biomarkers. Int J Pharm Biol Arch. 2014;5:112. [Google Scholar]
- 18.Sezer E, Ozugurlu F, Ozyurt H, Sahin S, Etikan I. Lipid peroxidation and antioxidant status in lichen planus. Clin Exp Dermatol. 2007;32:430–4. doi: 10.1111/j.1365-2230.2007.02436.x. [DOI] [PubMed] [Google Scholar]
- 19.Hassan I, Keen A, Majid S, Hassan T. Evaluation of the antioxidant status in patients of lichen planus in Kashmir valley – A hospital based study. J Saudi Soc Dermatol Dermatol Surg. 2012;17:13–6. [Google Scholar]
- 20.Aly DG, Shahin RS. Oxidative stress in lichen planus. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:3–11. [PubMed] [Google Scholar]
- 21.Jingyan Z, Ming T, Yunhai D. Changes of serum SOD and LPO levels in OLP patients before and after the therapy integrated with traditional Chinese medication. Acta Acad Med Wannan. 2000-2001;5:5–9. [Google Scholar]
- 22.Agha-Hosseini F, Mirzaii-Dizgah I, Mikaili S, Abdollahi M. Increased salivary lipid peroxidation in human subjects with oral lichen planus. Int J Dent Hyg. 2009;7:246–50. doi: 10.1111/j.1601-5037.2009.00365.x. [DOI] [PubMed] [Google Scholar]
- 23.Cheng YS, Jordan L, Rees T, Chen HS, Oxford L, Brinkmann O, et al. Levels of potential oral cancer salivary mRNA biomarkers in oral cancer patients in remission and oral lichen planus patients. Clin Oral Investig. 2014;18:985–93. doi: 10.1007/s00784-013-1041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agha-Hosseini F, Mirzaii-Dizgah I, Farmanbar N, Abdollahi M. Oxidative stress status and DNA damage in saliva of human subjects with oral lichen planus and oral squamous cell carcinoma. J Oral Pathol Med. 2012;41:736–40. doi: 10.1111/j.1600-0714.2012.01172.x. [DOI] [PubMed] [Google Scholar]
- 25.Mishra SS, Uma Maheswari TN. Evaluation of oxidative stress in oral lichen planus using malonaldehyde: A systematic review. J Saudi Soc Dermatol Dermatol Surg. 2014;7:14–20. [Google Scholar]
- 26.Rai B, Kharb S, Jain R, Anand SC. Salivary lipid peroxidation product malonaldehyde in pre-cancer and cancer. Adv Med Dent Sci. 2008;2:7–8. [Google Scholar]
- 27.Upadhyay RB, Carnelio S, Shenoy RP, Gyawali P, Mukherjee M. Oxidative stress and antioxidant defense in oral lichen planus and oral lichenoid reaction. Scand J Clin Lab Invest. 2010;70:225–8. doi: 10.3109/00365511003602455. [DOI] [PubMed] [Google Scholar]
- 28.Scrobota I, Mocan T, Filip A, Daicoviciu D, Baciut G. Oxidants-antioxidants balance in oral lichen planus. Fiziologia. 2010;20:27–31. [Google Scholar]
- 29.Abbas AW, Zaidan TF, Abduladheem Y, Al-Barrak A. Assessment of serum and salivary malondialdehyde in patients with oral lichen planus. J Bagh Coll Dent. 2014;26:99–102. [Google Scholar]
- 30.Shirzad A, Pouramir M, Seyedmajidi M, Jenabian N, Bijani A, Motallebnejad M, et al. Salivary total antioxidant capacity and lipid peroxidation in patients with erosive oral lichen planus. J Dent Res Dent Clin Dent Prospects. 2014;8:35–9. doi: 10.5681/joddd.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–86. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–55. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barikbin B, Yousefi M, Rahimi H, Hedayati M, Razavi SM, Lotfi S, et al. Antioxidant status in patients with lichen planus. Clin Exp Dermatol. 2011;36:851–4. doi: 10.1111/j.1365-2230.2011.04152.x. [DOI] [PubMed] [Google Scholar]
- 34.Scrobotă I, Mocan T, Cătoi C, Bolfă P, Mureşan A, Băciuţ G, et al. Histopathological aspects and local implications of oxidative stress in patients with oral lichen planus. Rom J Morphol Embryol. 2011;52:1305–9. [PubMed] [Google Scholar]
- 35.Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond) 2003;105:167–72. doi: 10.1042/CS20030031. [DOI] [PubMed] [Google Scholar]
- 36.Al-Rawi N, Jaber F, Atiyah K. Assessment of salivary and serum oxidative stress and antioxidants as plausible parameters in prediction of ischemic stroke among Iraqi Samples. Internet J Third World Med. 2008;7:2–8. [Google Scholar]
- 37.Saxena S. Assessment of plasma and salivary antioxidant status in patients with recurrent aphthous stomatitis. RSBO. 2011;8:261–5. [Google Scholar]
- 38.Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diab Vasc Dis Res. 2011;8:22–8. doi: 10.1177/1479164110390243. [DOI] [PubMed] [Google Scholar]
- 39.Soukup M, Biesiada I, Henderson A, Idowu B, Rodeback D, Ridpath L, et al. Salivary uric acid as a noninvasive biomarker of metabolic syndrome. Diabetol Metab Syndr. 2012;4:14. doi: 10.1186/1758-5996-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


