Abstract
Introduction:
Oral submucous fibrosis (OSMF), a precancerous condition, is highly prevalent in the Indian subcontinent. Among many trace elements, copper and iron are required for the functioning of numerous enzymes. The biochemical alterations of these trace elements in the serum of patients with premalignant conditions can help in determining the staging of the disease, its appropriate treatment and as an indicator for prognosis.
Aims and Objectives:
The objective of the study was to evaluate the role of iron and copper as well as to identify the better predictor of the two in the diagnosis and progression of OSMF.
Materials and Methods:
The study sample consisted of 150 patients, out of which the cases group consisting of 100 OSMF patients and control group consisting of 50 individuals. All the cases were confirmed of having OSMF by histopathological examination. The blood sample was obtained from all 150 patients and evaluated by digital autoanalyzer photometer for serum level of copper and iron by the colorimetric method. The results obtained for cases and controls were compared by statistical analysis.
Results:
The mean serum copper level increases while the mean serum iron level decreases with the advancement in the severity of clinical and histological stages of OSMF.
Conclusion:
Biopsy is the gold standard to diagnose OSMF, but it is an invasive and time-consuming technique. However, nowadays, many recent advances are used to diagnose OSMF at an early stage and reduce its progression into late and reversible stages. The trace elements serve as potential prognostic and diagnostic markers for OSMF patients.
Keywords: Copper and Iron, oral submucous fibrosis, precancerous condition
INTRODUCTION AND REVIEW OF LITERATURE
Oral submucous fibrosis (OSMF) is a chronic, precancerous condition, found to affect the South and South East Asian population, especially those of the Indian subcontinent.[1,2,3,4,5,6,7] It has now become an Indian epidemic with an estimated 2.5 million people being affected with this disease.[8,9]
It has been suggested that areca nut chewing, consumption of chillies, genetic susceptibility, nutritional deficiency, autoimmunity and collagen disorders may be involved in the pathogenesis of this condition. The most common etiology considered for causation of OSMF is “arecoline” which is a constituent of areca nut.[1,4,5,9,10,11,12,13,14,15,16,17,18]
In the early cases of OSMF, oral mucosa becomes blanched and slightly opaque.[10,19,20,21] Fibrosis of mucosa occurs in late cases of OSMF, leading to stiffness, most commonly in palate, buccal mucosa and faucial pillars. With progressive fibrosis, there is difficulty in opening the mouth, inability to whistle or blow out a candle and sometimes difficulty in swallowing.[4,10,19,20]
Histopathologically, early cases of OSMF demonstrate epithelial hyperplasia, thickened collagen bundles, moderate numbers of large fibroblasts and inflammatory cell infiltration containing a number of polymorphonuclear leukocytes.[9,12] In advanced cases, epithelial atrophy, dense bundles and sheets of collagen, thick bands of subepithelial hyalinization extending into the submucosal tissues (replacing fat or fibrovascular tissue), decreased vascularity, no edema and decreased inflammatory cells (lymphocytes and plasma cells) are found.[9,10,12,19,20,22]
OSMF is a well-recognized, potentially premalignant condition. Malignant transformation rate as high as 7.6% has been reported from the Indian subcontinent over a 17 year period.[5,6,8,9,12,23,24,25,26,27,28,29]
Biopsy is a gold standard for establishing diagnosis and prognosis of OSMF. It is an invasive, time-consuming procedure and causes psychological trauma to some patients. Thus, the need of the hour is that the test ought to be simple, quick, least invasive, easy to interpret, economical and yet quite confirmatory for its diagnosis. Hence, other diagnostic procedures such as hematological profile, trace element analysis, circulating immune complexes, cytochemical markers and cytogenetic studies have also been used.[30]
Among many trace elements, copper and iron are required for the functioning of numerous enzymes. Copper is an important trace element found in human body. It forms a part of important enzymes such as cytochrome, C oxidase, superoxide dismutase, metallothionein and lysyl oxidase.[15,25,27,30,31,32,33] Iron has been recognized as an important element for maturation of epithelium, and it is present in many hemoproteins such as hemoglobin, myoglobin and cytochrome.[15,25,30] Thus, biochemical alterations of copper and iron concentrations in the serum of premalignant patients can help in determining the staging of the disease, its appropriate treatment and as an indicator for prognosis.[6,15,25,26,27,30,32,33]
The present study is aimed to estimate and compare the levels of serum copper and iron among individuals with OSMF of different clinical and histological stages.
MATERIALS AND METHODS
The study sample consisted of 50 patients without OSMF (controls) and 100 OSMF patients (cases) coming to Dental Outpatient Department of Department of Oral Medicine and Radiology of the dental institute. The cases were included in the study having either habit of eating spicy food and/or chewing areca nut/tobacco or having two or more of the following signs and symptoms suggestive of OSMF:
Burning sensation and difficulty in eating hot and spicy food
Reduced mouth opening
Blanched or opaque appearance of the mucosa.
Individuals were then interviewed and the history findings were recorded in a case sheet pro forma, especially prepared for the study. After thorough history taking was completed, patients were subjected to clinical examination after taking written consent. Patients were categorized into different clinical stages as per the criteria of Andrade and Khanna's staging system.[34]
After clinical examination and staging, a punch biopsy [Figures 1 and 2] was taken from buccal mucosa and tissue was sent to Oral Pathology Department of the institute for detailed histopathological examination. Histological grading was done as per the criteria of Khanna and Andrade grading system [Figures 3–6].[34] The study was conducted as per the IRB guidelines and Helsinki Declaration and after approval from the ethical committee of the institute.
Figure 1.
Armamentarium used for taking punch biopsy
Figure 2.
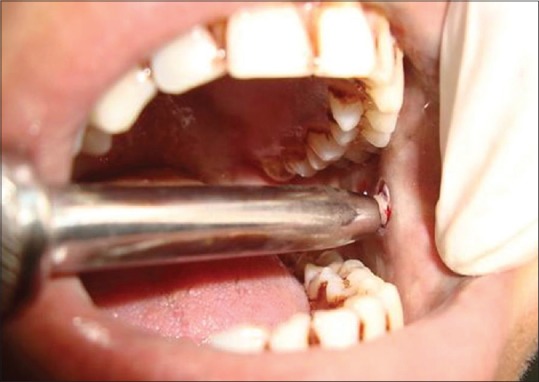
Procedure of taking punch biopsy from buccal mucosa
Figure 3.

Features of histopathological Grade I oral submucous fibrosis in ×10 magnification
Figure 6.
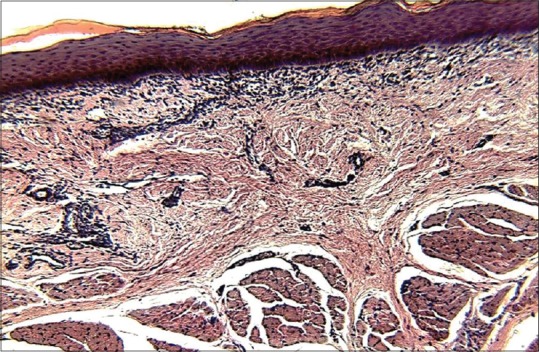
Features of histopathological Grade IV oral submucous fibrosis in ×10 magnification
Figure 4.
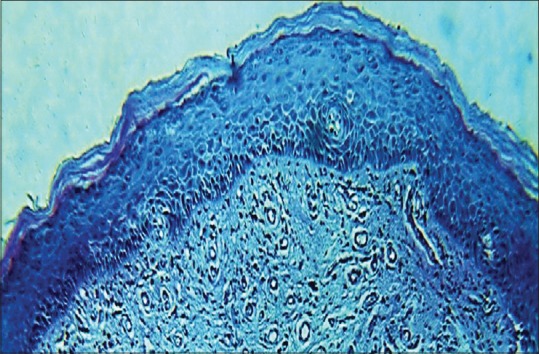
Features of histopathological Grade II oral submucous fibrosis in ×10 magnification
Figure 5.
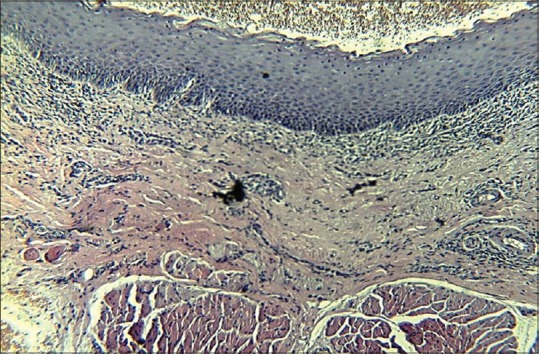
Features of histopathological Grade III oral submucous fibrosis in ×10 magnification
Estimation of serum copper and iron was done by collecting 5 ml of venous blood from median cubital vein under aseptic precautions with 5 ml syringe and 22-gauge needle [Figure 7]. The blood was allowed to clot and then centrifuged to provide serum. This serum was preserved in a frozen state until analysis. Preserved serum samples used for the estimation were mixed with appropriate proportions of buffer and color reagents and the absorbance of these samples was measured with a digital autoanalyzer photometer to estimate copper and iron content by the colorimetric method [Figure 8].
Figure 7.
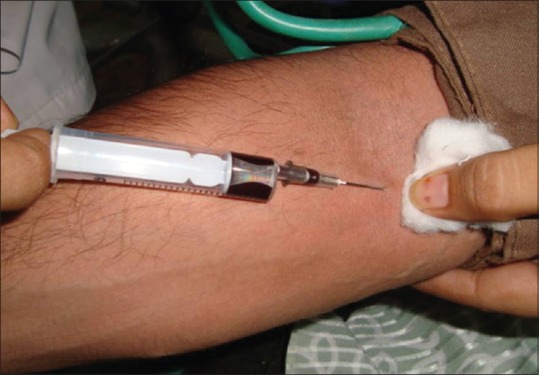
Procedure for collecting blood sample
Figure 8.

Digital autoanalyzer photometer
Data analysis was done using SPSS version 12.0 for Windows (SPSS, Inc., Chicago, IL, USA). The correlation between clinical staging and histological grading was confirmed statistically by Pearson's correlation. The differences between the mean serum copper and iron levels of study and control group were statistically tested using independent t-test. The differences between the mean serum copper/iron levels in different clinical stages/histological grades were statistically tested by analysis of variance (ANOVA) test.
RESULTS
Table 1 demonstrates comparison between histological grading and clinical staging in OSMF patients. Out of 100 OSMF patients, 3 belonged to histological Grade I, 34 belonged to histological Grade II, 43 belonged to histological Grade III and 20 belonged to histological Grade IV. Out of 4 patients of clinical Stage I, 3 (75%) patients belonged to histological Grade I and 1 (25%) patient belonged to histological Grade II. Out of 36 patients of clinical Stage II, 28 (77.8%) patients belonged to histological Grade II, followed by 7 (19.4%) patients in Grade III and 1 (2.8%) patient in histological Grade IV. Out of 44 patients of clinical Stage III, 33 (75%) patients belonged to histological Grade III, followed by 6 (13.7%) patients in histological Grade IV and 5 (11.5%) patients in histological Grade II. Out of 16 patients of clinical Stage IV, 13 (81.2%) patients belonged to histological Grade IV, followed by 3 (18.8%) patients in histological Grade III. Pearson's correlation test revealed that r = 0.792.
Table 1.
Comparison between histological grading and clinical staging in oral submucous fibrosis patients
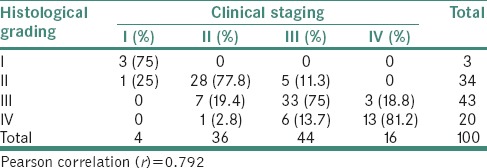
Table 2 demonstrates comparison of serum copper and iron concentrations (μg/ml) in individuals of study group and control group. Patients of study group exhibited higher mean copper (153.2 ± 13.1) and lower mean iron (38.8 ± 12.2) levels in contrast to controls. Independent t-test revealed that P < 0.01.
Table 2.
Comparison of mean serum copper and iron concentrations (μg/ml) in individuals of study group and control group
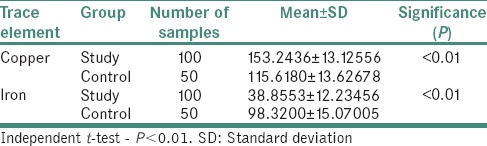
Table 3 demonstrates relation between clinical staging and serum copper level (μg/ml) in OSMF patients. The mean serum copper value of 100 OSMF patients was 153.24 ± 13.12 with minimum value being 119.5 and maximum value being 179.57. The mean serum copper value for clinical Stages I, II, III and IV was, respectively, 136.13 ± 13.2, 147.64 ± 9.58, 155.41 ± 12.87 and 164.16 ± 10.85. ANOVA test revealed that P < 0.01.
Table 3.
Relation between clinical staging and serum copper level (μg/ml) in oral submucous fibrosis patients
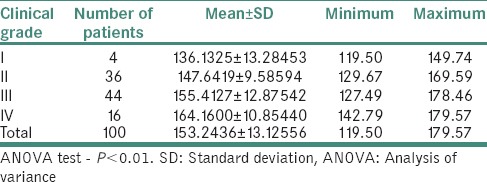
Table 4 demonstrates the relation between histological grading and serum copper level (μg/ml) in OSMF patients. The mean serum copper value of 100 OSMF patients was 153.24 ± 13.12 with minimum value being 119.5 and maximum value being 179.57. The mean serum copper value for histological Grade I, II, III and IV was, respectively, 137.5 ± 15.9, 149.4 ± 11.05, 153.43 ± 13.85 and 161.73 ± 9.65. ANOVA test revealed that P < 0.01.
Table 4.
Relation between histological grading and serum copper level (μg/ml) in oral submucous fibrosis patients
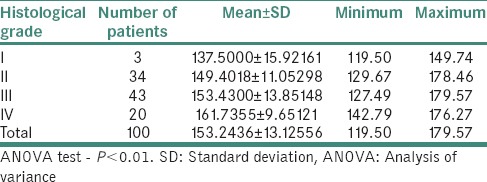
Table 5 demonstrates relation between clinical staging and serum iron level (μg/ml) in OSMF patients. The mean serum iron value of 100 OSMF patients was 38.85 ± 12.23 with minimum value being 16.27 and maximum value being 76.05. The mean serum iron value for clinical Stage I, II, III and IV was, respectively, 55.33 ± 19.5, 46.20 ± 9.10, 35.66 ± 9.99 and 26.96 ± 7.01. ANOVA test revealed that P < 0.01.
Table 5.
Relation between clinical staging and serum iron level (μg/ml) in oral submucous fibrosis patients
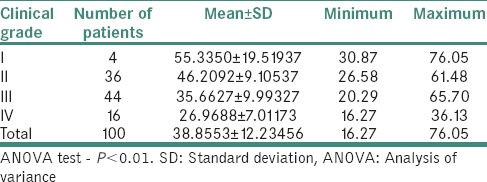
Table 6 demonstrates relation between histological grading and serum iron level (μg/ml) in OSMF patients. The mean serum iron value of 100 OSMF patients was 38.85 ± 12.23 with minimum value being 16.27 and maximum value being 76.05. The mean serum iron value for histological Grade I, II, III and IV was, respectively, 52.25 ± 22.68, 45.46 ± 10.56, 37.50 ± 10.42 and 28.51 ± 8.27. ANOVA test revealed that P < 0.01.
Table 6.
Relation between histological grading and serum iron level (μg/ml) in oral submucous fibrosis patients
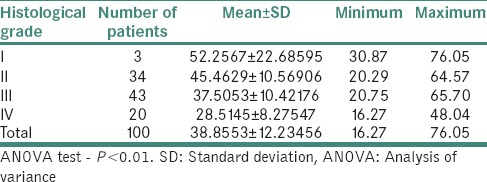
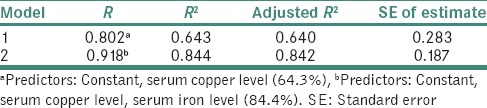
Table 7 demonstrates stepwise linear regression analysis with serum copper and iron levels as independent variables and individuals as dependent variable. The first best predictor was serum copper level which exerted an influence of 64.3% whereas serum copper and iron levels together exerted an influence of 84.4%.
Table 7.
Stepwise linear regression analysis with serum copper and iron levels as independent variables and groups (patients and controls) as dependent variable
There is a linear correlation between the mean serum copper level of OSMF patients and the severity of their clinical staging [Graph 1] as well as between the mean serum copper level of OSMF patients and the severity of their histological staging [Graph 2].
Graph 1.
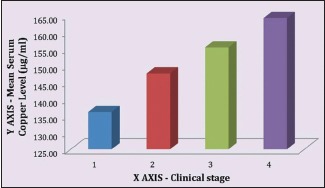
Mean serum copper level to clinical stages correlation in oral submucous fibrosis patients
Graph 2.
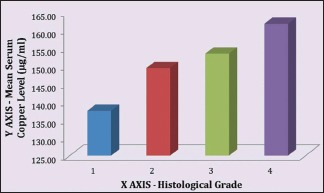
Mean serum copper level to histological stages correlation in oral submucous fibrosis patients
There is a linear correlation between the mean serum iron level of OSMF patients and the severity of their clinical staging [Graph 3] as well as between the mean serum iron level of OSMF patients and the severity of their histological staging [Graph 4].
Graph 3.
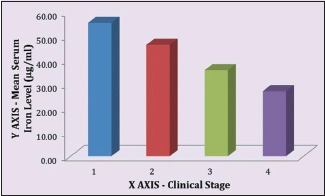
Mean serum iron level to clinical stages correlation in oral submucous fibrosis patients
Graph 4.
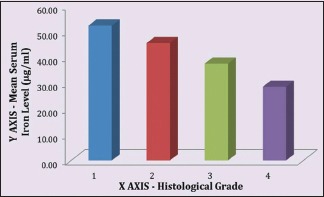
Mean serum iron level to histological stages correlation in oral submucous fibrosis patients
DISCUSSION
OSMF is a chronic, insidious and disabling condition of the oral mucosa characterized by epithelial atrophy and progressive accumulation of collagen fibers in the lamina propria and submucosa.[33] OSMF is a potentially malignant disease of oral cavity and is most commonly found in Asian countries. OSMF is considered a precancerous condition; it is essential to diagnose and treat as per the stage of the disease.[22]
Histopathological report is a gold standard for establishing diagnosis and prognosis of OSMF. Being an invasive and time-consuming procedure, there is a need for a quick and easy to interpret confirmatory investigation for diagnosis and prognosis of OSMF. Hence, other diagnostic procedures such as hematological profile, trace element analysis, circulating immune complexes, cytochemical markers and cytogenetic studies have also been used.[30] The present study was conducted to establish the correlation of serum copper and serum iron to the stages of OSMF.
It was observed that mean serum copper level was significantly high in cases group than in control group. Furthermore, a definite trend was observed with gradual increase in serum copper level as the clinical stage and histological grade of OSMF increased which is in accordance with the study of Tadakamadla et al.[6] Thus, there could be a direct relation between serum copper and extent of fibrosis.[6,15,26,30] Copper is an essential cofactor for expression of enzyme lysyl oxidase. High levels of copper in areca nut, a major etiological factor in OSMF, plays an initiating role in stimulation of fibrogenesis by upregulation of lysyl oxidase[11] and thereby causing inhibition of degradation of collagen. The rise in serum copper may be due to increased production of copper containing ceruloplasmin by liver as an inflammatory response or due to decrease in the catabolism of the serum ceruloplasmin. Khanna SS et al.[30] suggested that role of copper ions in biological damage is caused by superoxide radicals or other reducing agents such as ascorbate, which reduce the copper complex. It is seen that copper can upregulate the expression of the enzyme lysyl oxidase in the oral mucosa leading to fibrosis. One probable explanation is that copper stabilizes the enzyme activity by increasing its half-life. The other explanation is that at the molecular level, the N-terminus of exon-1 of lysyl oxidase molecule has copper binding site and this interaction may upregulate the expression of enzymes at cellular level. These events lead to cross-linking of collagen and elastin, making it less degradable.[6,15,24,26,27]
A significant trend was observed with gradual decrease in serum iron level as the clinical stage and histological grade of OSMF increased. Anuradha and Devi[25] have suggested that decreased iron levels in OSMF patients might be due to utilization of iron in collagen synthesis. Hydroxyproline is an amino acid found only in collagen. Iron is required for collagen synthesis by enzymes in hydroxylation of proline and lysine. This hydroxylation of proline and lysine is catalyzed by proline hydroxylase and peptidyl lysine hydroxylase, respectively. Peptidyl proline hydroxylase requires as cofactory molecular oxygen, ferrous iron, alpha-ketoglutarate and ascorbic acid.[30] The decreased iron level may be due to utilization in the fibrosis process. The presence of iron deficiency anemia could be attributed to the clinical nature of OSMF. The initial burning sensation, vesiculation and ulceration render the consumption of solid food, an unpalatable affair leading to poor consumption of normal diet that could possibly initiate the anemia. After frank establishment of the lesion, anemia may be further perpetuated by the inadequate intake of food due to burning sensation, fibrosis and trismus. It is well-established fact that epithelial atrophy and lack of proper maturation of epithelium are two important histological changes observed in the oral mucosa of patients with iron deficiency anemia. Further, lack of iron in the tissues also results in decreased vascularity. The normal maturation of epithelium is dependent on an iron-containing enzyme called cytochrome oxidase. In iron deficiency anemia, levels of this enzyme are low and a consequent atrophy of epithelium and lack of maturation results.[6,15,30,33]
Applying linear regression analysis with staging of lesions as dependent variable, the first best predictor was serum copper level which exerted an influence of 64.3% whereas serum copper and serum iron levels together exerted an influence of 84.4% which is in accordance with the findings of Tadakamadla et al.,[6] Luqaman and Vidya[15] and Khanna and Karjodkar.[30]
CONCLUSION
OSMF is a complex precancerous condition of the oral cavity and oropharynx. Once the fibrosis sets, the condition is irreversible and has a high malignant potential. Thus, vigorous steps should be taken for its early diagnosis, hence to improve its prognosis. Biopsy is the gold standard to diagnose OSMF, but it is an invasive and time-consuming technique. However, nowadays, many recent advances are used to diagnose OSMF in very early stage and reduce its progression into late and irreversible stages. It can be concluded from the present study that serum copper and iron levels could be used as a potential prognostic and diagnostic markers in OSMF patients. However, future studies are anticipated on a larger heterogeneous population to confirm the hypothesis. These efforts may be of value for proactive intervention of high-risk groups and may also serve in predicting malignant potential of other premalignant lesions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tupkari TV, Bhavthankar JD, Mandale MS. Oral submucous fibrosis (OSMF): A study of 101 cases. J Indian Acad Oral Med Radiol. 2007;19:311–8. [Google Scholar]
- 2.Bose T, Balan A. Oral submucous fibrosis – A changing scenario. J Indian Acad Oral Med Radiol. 2007;19:334–40. [Google Scholar]
- 3.Merchant AT, Haider SM, Fikree FF. Increased severity of oral submucous fibrosis in young Pakistani men. Br J Oral Maxillofac Surg. 1997;35:284–7. doi: 10.1016/s0266-4356(97)90049-8. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad MS, Ali A, Ali AS, Chaubey KK. Epidemiological and etiological study of oral submucous fibrosis among gutkha chewers of Patna, Bihar, India. J Indian Soc Pedod Prev Dent. 2006;24:84–9. doi: 10.4103/0970-4388.26022. [DOI] [PubMed] [Google Scholar]
- 5.Kumar K, Saraswathi TR, Ranganathan K, Devi U, Elizabeth J. Oral submucous fibrosis: A clinic-histopathological study in Chennai. Indian J Dent Res. 2007;18:106–11. doi: 10.4103/0970-9290.33785. [DOI] [PubMed] [Google Scholar]
- 6.Tadakamadla J, Kumar S, Mamatha GP. Evaluation of serum copper and iron levels among oral submucous fibrosis patients. Med Oral Patol Oral Cir Bucal. 2011;3:1–4. doi: 10.4317/medoral.17083. [DOI] [PubMed] [Google Scholar]
- 7.Shah N, Kumar R, Shah MK. Immunological studies in oral submucous fibrosis. Indian J Dent Res. 1994;5:81–7. [PubMed] [Google Scholar]
- 8.Eipe N. The chewing of betel quid, oral submucous fibrosis and anaesthesia. Anesth Analog. 2005;100:1210–3. doi: 10.1213/01.ANE.0000146434.36989.34. [DOI] [PubMed] [Google Scholar]
- 9.Pillai R, Balaram P, Reddiar KS. Pathogenesis of oral submucous fibrosis: Relationship to risk factors associated with oral cancer. Cancer. 1992;69:2011–20. doi: 10.1002/1097-0142(19920415)69:8<2011::aid-cncr2820690802>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Rajendran R. Oral submucous fibrosis. J Oral Maxillofac Pathol. 2003;7:1–4. doi: 10.4103/0973-029X.86678. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Pindborg JJ, Mehta FS, et al. Etiology of oral submucous fibrosis with special reference to the role of areca nut chewing. J Oral Pathol Med. 1995;24:145–52. doi: 10.1111/j.1600-0714.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 12.Canniff JP, Harvey W, Harris M. Oral submucous fibrosis: Its pathogenesis and management. Br Dent J. 1986;160:429–34. doi: 10.1038/sj.bdj.4805876. [DOI] [PubMed] [Google Scholar]
- 13.Mathew AL, Pai KM, Sholapurkar AA, Vengal M. The prevalence of oral mucosal lesions in patients visiting a dental school in Southern India. Indian J Dent Res. 2008;19:99–103. doi: 10.4103/0970-9290.40461. [DOI] [PubMed] [Google Scholar]
- 14.Ranganathan K, Mishra G. An overview of classification schemes for oral submucous fibrosis. J Oral Maxillofac Pathol. 2006;10:55–8. [Google Scholar]
- 15.Luqaman M, Vidya V. The role of serum copper and iron in oral submucous fibrosis. J Indian Acad Oral Med Radiol. 2004;16:30–2. [Google Scholar]
- 16.Metkari S, Tupkari JV, Barpande SR. An estimation of serum malondialdehyde, superoxide dismutase and vitamin A in oral submucous fibrosis and its clinic-pathologic correlation. J Oral Maxillofac Pathol. 2007;11:23–7. [Google Scholar]
- 17.Koshti S, Barpande S. Quantification of plasma fibrinogen degradation products in oral submucous fibrosis: A clinic-pathologic study. J Oral Maxillofac Pathol. 2007;11:48–50. [Google Scholar]
- 18.Hayes PA. Oral submucous fibrosis in a 4-year-old girl. Oral Surg Oral Med Oral Pathol. 1985;59:475–8. doi: 10.1016/0030-4220(85)90087-8. [DOI] [PubMed] [Google Scholar]
- 19.Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22:764–79. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 20.Rajendran R. Oral submucous fibrosis: Etiology, pathogenesis, and future research. Bull World Health Organ. 1994;72:985–96. [PMC free article] [PubMed] [Google Scholar]
- 21.Zain RB, Ikeda N, Gupta PC, Warnakulasuriya S, van Wyk CW, Shrestha P, et al. Oral mucosal lesions associated with betel quid, areca nut and tobacco chewing habits: Consensus from a workshop held in Kuala Lumpur, Malaysia, November 25-27, 1996. J Oral Pathol Med. 1999;28:1–4. doi: 10.1111/j.1600-0714.1999.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 22.Mani NJ, Singh B. Studies on oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 1976;41:203–14. doi: 10.1016/0030-4220(76)90232-2. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh PK, Madhavi R, Guntur M, Ghosh R. Sister chromatid exchanges in patients with oral submucous fibrosis. Cancer Genet Cytogenet. 1990;44:197–201. doi: 10.1016/0165-4608(90)90047-e. [DOI] [PubMed] [Google Scholar]
- 24.Trivedy C, Meghji S, Warnakulasuriya KA, Johnson NW, Harris M. Copper stimulates human oral fibroblasts in vitro: A role in the pathogenesis of oral submucous fibrosis. J Oral Pathol Med. 2001;30:465–70. doi: 10.1034/j.1600-0714.2001.030008465.x. [DOI] [PubMed] [Google Scholar]
- 25.Anuradha CD, Devi CS. Studies on the hematological profile and trace elements in oral submucous fibrosis. J Clin Biochem Nut. 1995;19:9–17. [Google Scholar]
- 26.Shettar SS, Mubeen AS. Estimation of serum copper and zinc levels in patients with oral submucous fibrosis. J Indian Acad Oral Med Radiol. 2010;22:193–6. [Google Scholar]
- 27.Trivedy CR, Warnakulasuriya KA, Peters TJ, Senkus R, Hazarey VK, Johnson NW, et al. Raised tissue copper levels in oral submucous fibrosis. J Oral Pathol Med. 2000;29:241–8. doi: 10.1034/j.1600-0714.2000.290601.x. [DOI] [PubMed] [Google Scholar]
- 28.Shieh DH, Chiang LC, Shieh TY. Augmented mRNA expression of tissue inhibitor of metalloproteinase-1 in buccal mucosal fibroblasts by arecoline and safrole as a possible pathogenesis for oral submucous fibrosis. Oral Oncol. 2003;39:728–35. doi: 10.1016/s1368-8375(03)00101-5. [DOI] [PubMed] [Google Scholar]
- 29.Afroz N, Hasan SA, Naseem S. Oral submucous fibrosis: A distressing disease with malignant potential. Indian J Community Med. 2006;31:270–1. [Google Scholar]
- 30.Khanna SS, Karjodkar FR. Circulating immune complexes and trace elements (Copper, iron and selenium) as markers in oral precancer and cancer: A randomised, controlled clinical trial. Head Face Med. 2006;2:33. doi: 10.1186/1746-160X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandya S, Chaudhary AK, Singh M, Singh M, Mehrotra R. Correlation of histopathological diagnosis with habits and clinical findings in oral submucous fibrosis. Head Neck Oncol. 2009;1:10. doi: 10.1186/1758-3284-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanna S. Immunological and biochemical markers in oral carcinogenesis: The public health perspective. Int J Environ Res Public Health. 2008;5:418–22. doi: 10.3390/ijerph5050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taneja L, Anjana B, Vaishali K. Haemoglobin levels in patients with oral submucous fibrosis. J Indian Acad Oral Med Radiol. 2007;19:329–33. [Google Scholar]
- 34.Khanna JN, Andrade NN. Oral submucous fibrosis: A new concept in surgical management. Int J Oral Maxillofac Surg. 1995;24:433–9. doi: 10.1016/s0901-5027(05)80473-4. [DOI] [PubMed] [Google Scholar]


