Abstract
The hygiene or “Old Friends” hypothesis proposes that the epidemic of inflammatory disease in modern urban societies stems at least in part from reduced exposure to microbes that normally prime mammalian immunoregulatory circuits and suppress inappropriate inflammation. Such diseases include but are not limited to allergies and asthma; we and others have proposed that the markedly reduced exposure to these old friends in modern urban societies may also increase vulnerability to neurodevelopmental disorders and stress-related psychiatric disorders, such as anxiety and affective disorders, where data are emerging in support of inflammation as a risk factor. Here we review recent advances in our understanding of the potential for old friends, including environmental microbial inputs, to modify risk for inflammatory disease, with a focus on neurodevelopmental and psychiatric conditions. We highlight potential mechanisms, involving bacterially-derived metabolites, bacterial antigens, and helminthic antigens, through which these inputs promote immunoregulation. Though findings are encouraging, significant human subjects research is required to evaluate the potential impact of old friends, including environmental microbial inputs, on biological signatures and clinically meaningful mental health prevention and intervention outcomes.
Keywords: anxiety, depression, lactobacilli, microbiome, mycobacteria, posttraumatic stress disorder
Introduction
It is firmly established that inflammation can play a causative role in psychiatric disorders [reviewed in 1]. Here we summarize major points from the human data, but there is also a mass of animal data that falls outside the scope of this review. Raised levels of cytokines are found in the blood and cerebrospinal fluid (CSF) of a subset of cases of depression and psychosis [1-3], and raised expression is found in the brains of suicide victims [1]. Moreover depression is associated with polymorphisms of inflammatory cytokine genes [4], and the risk alleles tend to have inflammatory functions within the immune system [5]. The known environmental risk factors are also proinflammatory [6]. Recently, using non-invasive positron emission tomography (PET) scans, it has been possible to confirm that there is activation of microglia (tissue-resident macrophages in the central nervous system (CNS)) in depressed and in psychotic subjects [7;8]. Very direct evidence for the contributory role of inflammation comes from the observation that some patients become depressed when treated with IFN-alpha, an inflammatory cytokine [9]. Finally, antiinflammatory treatments such as inhibitors of cycloxygenase, or a neutralizing antibody to tumor necrosis factor (TNF) are therapeutic in those patients who have raised inflammatory biomarkers [10;11]. (The same subset tends to be resistant to conventional antidepressants [12]). Finally, it has been shown in rodents and monkeys that inflammation occurring during pregnancy can lead to abnormalities of brain development and behavior that are reminiscent of autism or schizophrenia [13;14], while epidemiological findings suggest that the same is true in humans [15].
A second class of evidence that inflammation can play a causative role in psychiatric disorders comes from studies of the pathways that link peripheral inflammation to changes in CNS function [1]. Cytokines can 1) access the brain via the circumventricular organs where there is a reduced blood-brain barrier, 2) be transported into the brain by specific transport mechanisms, or 3) cause inflammatory changes in the cells of the blood-brain barrier [16]. Other signals are transmitted via the vagus nerve which, in response to inflammatory signals in the periphery, drives cytokine release in the CNS [16]. Peripheral inflammation also activates enzymes such as indoleamine 2,3-dioxygenase that convert tryptophan to N-formylkynurenine, which is then converted to kynurenine. Kynurenine is raised in the plasma of depressed suicide attempters [17], and, following entry into the brain, it is converted into several metabolites with potent neuropsychiatric effects [18]. Meanwhile peripheral TNF somehow drives brain microglia to secrete chemokine (C-C motif) ligand 2 (CCL2; also referred to as monocyte chemoattractant protein 1 (MCP1), which attracts monocytes into the brain [19].
Against this background we now consider how microbial exposures, including environmental microbial exposures, influence the regulation of our immune systems in the context of the effects of inflammation on CNS function.
The “Old Friends” hypothesis and mental health
The epidemic of inflammatory disease, including allergy and asthma, in modern urban societies is increasing dramatically, but underlying biological mechanisms are still unexplained. The hygiene or “Old Friends” hypothesis proposes that this epidemic is due at least in part to reduced exposure to environmental microorganisms that normally prime immunoregulatory circuits and suppress inappropriate inflammation [20]. We and others have proposed that reduced exposure to these old friends in modern urban societies may increase vulnerability to neurodevelopmental disorders (including autism spectrum disorder (ASD), schizophrenia), and stress-related psychiatric disorders such as anxiety and mood disorders [21-38]. Exaggerated inflammation is emerging as an important risk factor in all of these disorders [21-38]. Immunoregulation, measured as a balanced expansion of effector T cell (also called helper T (Th) cell) populations and regulatory T cells (Treg), is known to be driven by microbial signals. These signals originate mainly from organisms with which mammals co-evolved, including: 1) the symbiotic microbiota residing in the cutaneous and mucosal surfaces (e.g., surfaces of the upper airways, lungs, and gastrointestinal tract); 2) pathogens associated with the “Old Infections” (for example, helminths) that were present throughout life in evolving human hunter-gatherer populations; and 3) organisms from the natural environment with which humans were inevitably in daily contact through inhalation or ingestion (and so had to be tolerated by the immune system) [25]. Immunoregulation is thought to be compromised in developed countries because of reduced contact with these three categories of organisms that are necessary for regulation, in the same way that removal of other reliably experienced developmental resources such as sunlight or vitamins cause a range of physiological problems [25]. Here we explore our rapidly expanding understanding of the potential role for compromised immunoregulation in allergies, neurodevelopmental disorders, and stress-related psychiatric disorders, and the diverse mechanisms through which exposure to the old friends may increase immunoregulation and suppress inappropriate inflammation.
Comorbidity of allergy, anxiety, and affective disorders
The hygiene hypothesis originated in epidemiologic studies of allergy. As originally envisaged by Strachan [39], the hygiene hypothesis proposed that lower incidence of infection in early childhood could be an explanation for the rapid rise in allergic diseases, such as asthma and hay fever, documented during the 20th century. Allergic or atopic disorders including allergic rhinitis, allergic asthma, eczema, and food allergies are prevalent and potentially disabling conditions [40]. These disorders are all increasing in prevalence in developed countries [40]. The “Old Friends” hypothesis was proposed to emphasize that we no longer believe that exposure to childhood infections or outright pathogens per se is beneficial but rather that lack of exposure to symbiotic organisms, as well as the “Old Infections”, is harmful [20]. Indeed, hygiene is important for prevention of serious infections. Epidemiological and clinical studies report a high incidence of anxiety and increased levels of emotional reactivity in individuals suffering from allergies [41-46]. This comorbidity is evidenced by the fact that the incidence of anxiety disorders among individuals with allergies is more than double with respect to the general population [41;44;47;48]. Moreover, studies in mice report that intranasal (i.n.) or aerosolized allergen exposure in sensitized animals results in the activation of limbic brain regions and anxiety-like behavioral responses [49;50]. While these studies have not quantified normalized antigenic exposures from airborne allergens, they suggest a strong association between allergy and anxiety disorders, consistent with a potential role for inadequate immunoregulation and exaggerated inflammation in both conditions.
Inadequate immunoregulation as a risk factor for neurodevelopmental and neuropsychiatric disorders
Results from animal models and clinical studies are consistent with the idea that inadequate immunoregulation increases risk for development of neurodevelopmental disorders, including ASD [51-54]. Elevated serum levels of the proinflammatory cytokine, interleukin (interleukin)-17A (IL-17A, produced by IL-17-expressing T cells, called Th17 cells, as well as a number of families of innate lymphoid cells (ILCs)) [55], have been noted in children with autism and were furthermore associated with symptom severity [53]. Th17 cells are involved in infection, autoimmunity, and inflammation, especially in skin or mucosal surfaces of the lungs and gut [56;57]. A Th17 bias may emerge during fetal development, as recent studies in mice have shown that maternal retinoic acid receptor–related orphan nuclear receptor γt (RORγt)–dependent Th17 cells and IL-17A mediate abnormal cortical development and an autism-like phenotype of offspring in a maternal immune activation model [51].
Importantly, for the thesis of this review, recent studies have demonstrated that the gut microbiota has the capacity to induce a specific population of Treg, the RORγt+ subset of Treg [58;59]. Loss of RORγ+ (there are two isoforms of RORγ, RORγt and RORγ) Tregs results in enhanced production of interleukin 17 (IL-17) and interferon-γ in the colons of otherwise unchallenged mice, and increases in chemically-induced colitis, indicating a decreased ability of colonic Tregs lacking RORγ to regulate inflammatory responses [58]. In germ-free mice, introduction of specific bacterial species are sufficient to increase RORγ+ Tregs to levels observed in their conventionally raised specific pathogen-free counterparts [58]. Thus, Choi and colleagues [51] argue that therapeutic targeting of Th17 cells in vulnerable pregnant mothers may reduce the risk of bearing children with inflammation-dependent ASD-like phenotypes, and specific immunoregulatory bacteria have the ability to shift the balance of RORγ+ T cells from a Th17 to a Treg bias. A window of vulnerability may coincide with a predicted window of time during the late second trimester and early third trimester associated with increased risk of autism following maternal infections [60], maternal exposures to stressors [61], and maternal exposures to environmental pollutants [62]. This time frame coincides with a critical window of Treg development in the fetus, including the emergence, seeding, and expansion of Treg populations and Th17 cells (from 12 weeks of gestation until early postnatal development, with some continued development out to 2 years of age) [63]. Together with the finding that maternal stress is associated with an infant microbiota with a biological signature of increased potential for inflammation [64], immunoregulatory or antiinflammatory approaches may have value for both prevention and treatment of ASD. Consistent with this hypothesis, treatment with the antiinflammatory flavonoid, luteolin, results in a significant improvement in functioning in children with ASD [65], in association with decreases in plasma concentrations of biomarkers of inflammation [66].
Alterations in T cell number and function have also consistently been detected in patients with schizophrenia [67]. Researchers have subsequently proposed that maternal immune activation, induced by intrauterine infection and additional factors, could facilitate the preferential generation of Th17 cells and subsequent priming of neuroinflammatory processes, ultimately contributing to the neuroprogression in schizophrenia [68]. A meta-analysis found that treatment with nonsteroidal antiinflammatory drugs (NSAID) could be a potential treatment strategy to reduce symptom severity in schizophrenia [69]. Recent non-human primate studies using 1st and 2nd trimester infection with a viral mimic of double stranded RNA have demonstrated behavioral abnormalities in offspring resembling both ASD and schizophrenia [70;71]. Further, abnormal neuronal dendritic formations were characterized in association with the behavioral changes observed in the non-human primate model of maternal immune activation [72], suggesting developmental changes in offspring associated with inadequate immunoregulation during pregnancy. Thus, an argument can be made supporting a role for inflammatory insults, particularly Th17-driven inflammation in the skin or mucosal surfaces of the lungs and gut, as risk factors for neurodevelopmental and neuropsychiatric disorders. Furthermore, an argument can be made that prophylactic exposure of the mother to Treg-promoting and Th17-reducing commensal or environmental bacteria may reduce that risk.
Inadequate immunoregulation as a risk factor for stress-related mental health disorders
Human and animal studies are consistent with the hypothesis that inadequate immunoregulation increases risk for development of stress-related psychiatric disorders [25;73;74]. That is, chronic low-grade inflammation (a potential consequence of chronic infection, psychosocial stress, trauma, exposure to environmental pollutants or toxins, autoimmune processes, chronic allergy) is associated with increased risk for mental disorders [75;76]. In prospective studies, high baseline plasma concentration of C-reactive protein (CRP; an acute-phase protein and pattern recognition receptor that is induced in response to inflammation), measured before deployment in military personnel, was associated with increased likelihood of posttraumatic stress disorder (PTSD) symptoms after deployment, suggesting that inflammation before trauma exposure may predispose individuals to PTSD symptoms [73]. Similar findings were evident in a gene expression study in soldiers pre- and post-deployment (Breen et al. 2015), where results indicated that genes involved in networks of innate immunity and interferon signalling are overexpressed in PTSD cases pre-deployment, suggesting causality (Breen et al., 2015). Furthermore, increased circulating levels of the proinflammatory cytokine IL-6 immediately following trauma exposure predict the later development of PTSD symptoms [77]. Although not confirmed in all studies, low-grade inflammation has been associated with PTSD, as indicated by elevated serum CRP, IL-1β, and IL-6 [78-80]. Moreover, patients with PTSD show enhanced spontaneous secretion of IL-1β, IL-6 and tumor necrosis factor (TNF) by isolated peripheral blood mononuclear cells, which correlates with symptom severity [81;82]. Consistent with these findings, subjects with PTSD also have higher risk for autoimmune disease, including inflammatory bowel disease and rheumatoid arthritis [83], while genome-wide association studies in PTSD cohorts have revealed association with ANKRD55 [84], a gene associated with several autoimmune and inflammatory disorders, including multiple sclerosis [85;86], type 2 diabetes mellitus [87], celiac disease [88], and rheumatoid arthritis [89].
Similar findings have been documented for developing depressive symptoms. For example, a study of over 3,000 individuals showed that elevated baseline plasma CRP or IL-6 predicted cognitive symptoms of depression measured 12 years later [90]. Moreover, in a study published early in 2014, elevated plasma concentrations of IL-6 in 9-year old children predicted depressive symptoms measured at age 18 [91]. Therefore, it is reasonable that exposures to immunoregulatory or antiinflammatory bacteria may be useful for the prevention or treatment of symptoms of PTSD or major depression. Consistent with this hypothesis, the glucocorticoid antiinflammatory hydrocortisone decreases the risk of subsequent PTSD if administered immediately after trauma exposure [92]. Furthermore, treatment of depressed patients with standard antidepressants plus an antiinflammatory compound was reported to decrease depressive symptoms more than antidepressants with a placebo [11;93].
“Old Friends” with immunoregulatory potential
The previous paragraphs show how anxiety and affective disorders are comorbid with chronic inflammatory conditions, and are epidemiologically linked to persistent inflammation, even in the absence of any clinically apparent inflammatory condition. The latter situation is evidence of failing immunoregulation. Similarly, we also described how failing immunoregulation during pregnancy, in animals and humans, can lead to neurodevelopmental problems in the fetus mediated by excessive inflammation. We now discuss, within each of the microbiological categories that constitute the old friends, the organisms that have been shown to have immunoregulatory properties that might be exploited for prevention of, or as therapies for, psychiatric conditions.
Commensal or mutualistic microorganisms with immunoregulatory potential
Much of the focus on the interface between the human microbiome and potential positive human health outcomes has been put on commensal or mutualistic organisms, particularly those associated with the gut microbiome. Two recent studies have shed light on the potential for commensal gut microorganisms to influence immunoregulatory circuits [58;59]. These two studies demonstrated that the gut microbiota induces expansion of a specific subset of cells, known as the RORγt+ Treg in the lamina propria of the gastrointestinal tract. They then used chemical inhibitors and genetic manipulation to block the function of these Treg, and demonstrated that they have broad antiinflammatory effects in several models of colitis. They also noted that this cell type is severely depleted in germ-free or antibiotic-treated mice [58;59]. However, reconstituting the gut microbiota could restore expansion of these Treg. Importantly, oral administration of a single microbial species was sufficient to increase the frequency of RORγt+ Treg within the colon of germ-free mice, in some cases to levels observed in specific-pathogen-free control mice, which have a typical microbiota. Commensal bacteria that increase RORγt+ Treg include several species belonging to the phylum Firmicutes, a phylum with a large number of probiotic species, to include Clostridium histolyticum, C. ramosum, Enterococcus faecium, Lactobacillus casei, L. rhamnosus, and Staphylococcus saprophyticus. However, species from other phyla were also effective, including Bifidobacterium breve (Actinobacteria), Bacteroides thetaiotaomicron and Parabacteroides johnsonii (Bacteroidetes), Fusobacterium mortiferum, and F. nucleatum (Fusobacteria), and Acinetobacteria lwoffi (Proteobacteria). As mentioned above, loss of RORγ+ Tregs results in enhanced production of colonic IL-17 and interferon-γ and increases in chemically-induced colitis, indicating a decreased ability of colonic Tregs lacking RORγ to regulate inflammatory responses [58]. Thus, commensal bacteria from diverse phyla have the capacity to induce Treg (Table 1). Many of these same probiotic species have been shown to have stress-protective and mental health benefits in rodent and human studies (Table 1). The mechanisms through which bacteria from such diverse phyla can induce immunoregulatory pathways are not clear, but a number of candidate mechanisms are discussed below.
Table 1. Immunoregulatory microorganisms with beneficial effects in mental health studies.
| Phylum/ Microorganism |
Model | Environmental Sources |
Mental health relevant findings |
Immunoregulation |
|---|---|---|---|---|
| Actinobacteria | ||||
|
Mycobacterium
vaccae |
Human | Environmental saprophyte (soil, mud, water, grasses, decaying organic matter) [155-161] |
Increased cognitive function, decreased pain in patients with advanced non-small-cell lung cancer [151] |
↑DCreg [162] |
| Mouse | Activation of brain serotonergic systems and antidepressant-like behavioral effects [163]; decreased anxiety/increased cognitive function [164] |
↑Treg [110] | ||
| Promotion of proactive behavioral responses to psychosocial stress; prevention of stress- induced colitis and stress- induced exaggeration of chemically-induced colitis; reduction of anxiety-like behavior in stressed mice [152] | ||||
|
Bifidobacterium
breve |
Mouse | Human commensal |
Increased cognitive function [165]; decreased anxiety-related behaviors [166] |
↑Rorγ+ Helios− Treg [58] |
|
Bifidobacterium
infantis |
Rat | Human commensal |
Reversal of depressive- like behavior following maternal separation [167] |
↑Treg [168] |
|
Bifidobacterium
longum |
Human | Human commensal |
Decreased anxiety and depressive symptoms in healthy volunteers (administered with L. helveticus) [148;169] |
|
| Mouse | Decreased-colitis associated anxiety [170;171]; increased cognitive function (1714) [165]; decreased stress, anxiety- and depression- related behaviors [166] |
↑Treg and functional Treg responses [172] |
||
| Bacteroidetes | ||||
|
Bacteroides
fragilis |
Mouse | Human commensal |
Developmental protection from some of the behavioral symptoms associated with Autism Spectrum Disorder [54] |
↑Treg [173] |
| Firmicutes | ||||
|
Clostridium
butyricum |
Human | Endospore- forming soil bacterium |
Anxiolytic effects [149] | |
|
Enterococcus
faecium |
Mouse | Human commensal, wetlands [174] |
Increased brain antioxidant markers [175] |
|
|
Lactobacillus
casei |
Human | Human commensal, fermented foods [176] |
Improvement in anxiety symptoms in patients with chronic fatigue syndrome [177]; improved mood [178] |
↑Rorγ+ Helios− Treg [58] |
| Decreased perceived stress in medical students preparing for a nationwide exam [150] |
||||
|
Lactobacillus
fermentum |
Rat | Human commensal, raw vegetables [179], fermented foods [176;180] |
Decreased anxiety and inhibition of antibiotic- induced cognitive impairment [181] |
↑Treg [182] |
|
Lactobacillus
helveticus |
Human | Fermented foods [176] |
Decreased anxiety and depressive symptoms in healthy volunteers (administered with B. longum) [148;169]; increased cognitive function (IDCC3801) [183] |
|
| Rat | Improved cognitive function, decreased anxiety-related behavior [184]; prevention of stress-induced cognitive impairment and anxiety- and depressive-like responses [185] |
|||
| Mouse | Decreased anxiety-related behavior [186]; improved cognitive function, decreased anxiety-related behavior (administered with L. rhamnosus) [187;188] |
↑Treg (with L. rhamnosus [189] |
||
|
Lactobacillus
pentosus |
Mouse | Fermented foods [176] |
Improved cognitive function [190] |
|
|
Lactobacillus
reuteri |
Human | Human commensal, fermented foods [176] |
Increased workplace healthiness [191] |
↑Treg [192] |
|
Lactobacillus
rhamnosus |
Mouse | Human commensal, fermented foods [176] |
Vagus nerve dependent alterations in GABA receptor mRNA expression in brain, reduced anxiety- and depression-related behavior[193]; improved cognitive function, decreased anxiety-related behavior (administered with L. helveticus) [187;188] |
↑Rorγ+ Helios− Treg [58]; ↑Treg (with L. helveticus) [189] |
Immunoregulatory probiotics that induce Rorγ+Helios− Tregs, but have not been tested in the context of mental health, F. mortiferum, A. lwoffii, L. casei, E. faecium, F. nucleatum, P. johnsonii, L. rhamnosus, C. histolyticum, B. thetaiotaomicron, S. saprophyticus, C. ramosum [58].
Adapted from Hoisington et al. [31], with permission.
“Old Infections” with immunoregulatory potential
A number of “Old Infections” can induce immunoregulation. For example, humans co-evolved with Helicobacter pylori for tens of thousands of years. H. pylori is found in higher abundance in rural Papua New Guineans [94] and was recently found in previously uncontacted Amazonian Amerindians [95], as well as in the 5300-year old Iceman [96]. H. pylori is potently immunoregulatory, and consequently has protective effects in allergy and chronic inflammatory disorders [97;98]. Additional “Old Infections” include hepatitis A virus (HAV), Toxoplasma gondii (T. gondii), Salmonella, gut helminths and blood nematodes, and Mycobacterium tuberculosis [6;99]. A common feature of many of these “Old Infections” is that they bind the C-type lectin receptor, dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN; also referred to as cluster of differentiation 209 (CD209)) on dendritic cells (DC), in many cases using the receptor to gain entry into immune cells and subvert the host immune response [100-105]. Interactions between “Old Infections” and DC-SIGN can induce immunoregulatory responses [101;104], and the central feature of pathogens that can interact with DC-SIGN is that they cause chronic infections that can last a lifetime, persistence that is often dependent on a suppression of Th1 and concomitant shift to Th2 immunity [103].
Environmental microorganisms with immunoregulatory potential
In addition to commensal and mutualistic microorganisms and the “Old Infections”, environmental microorganisms also induce immunoregulation following ingestion [106] or inhalation [107;108]. These organisms have been referred to as “pseudocommensals” because they would have been present in large numbers throughout mammalian evolution, even if they do not colonize the gut [20;109]. Our understanding of the “Old Friends” hypothesis has been informed by studies using heat-inactivated and viable environmental bacteria, particularly soil-derived bacteria belonging to the genus Mycobacterium, which can be viewed as a case study of the potential for environmental microorganisms to influence immunoregulatory circuits and prevent inflammatory disease [110]. While it has long been believed that mycobacteria are not normally present in the human microbiome or the built environment in developed countries, recent studies reveal that a broad spectrum of Mycobacteria spp. are systemic residents of operating metropolitan water distribution systems [111;112]. To what extent these and other microbes impact humans through common infrastructure exposures, however, remains unknown. Another recent study has defined the hidden ‘non-tuberculous mycobacteriome’, with approximately 50 prevalent and abundant mycobacterial operational taxonomic units, in the nostrils, buccal mucosa, oropharynx, and dental plaque of healthy subjects [113], a distribution that may reflect the environmental origin of these microorganisms. Together, (1) disruption of the commensal microbes, (2) a reduction in “Old Infections”, and (3) diminished contact with immunoregulatory environmental microbes have the potential to increase vulnerability to allergic and inflammatory disorders, as well as mental health disorders, where chronic inflammation is emerging as an associated risk factor.
Diverse molecular mechanisms through which “Old Friends” induce antiinflammatory and immunoregulatory effects
Bacterially derived metabolites that induce immunoregulatory responses
Tryptophan and bacterially- and host-derived tryptophan metabolites
Evidence suggests that commensal bacteria are important for the extraction, synthesis or absorption of some amino acids, including tryptophan. In particular, plasma tryptophan and N-acetyltryptophan concentrations in conventionally reared mice are 40% and 60% lower, respectively, compared to germ-free mice [114], suggesting that the gut microbiota plays an important role in metabolism of tryptophan. A subset of enteric bacteria expresses tryptophanase, which converts tryptophan to indole, pyruvate, and ammonia. Meanwhile, it is becoming increasingly clear that tryptophan and diverse tryptophan metabolites (both bacterially-derived and host-derived) have immunomodulatory effects. Tryptophan influences proliferation of T cells by regulating passage through the gap 1 (G1) phase of the cell cycle [115]. Tryptophan depletion, secondary to activation of indoleamine-2,3-dioxygenase, regulates immune tolerance of the fetus and regulates immune responses in models of skin allograft rejection, tumor growth and autoimmune encephalomyelitis [116] and chemically-induced colitis [117]. The tryptophan metabolite melatonin induces Treg via actions on melatonin receptors (MT1) [118]. Interactions between bacterially-driven and host-driven tryptophan metabolism are important for host defense. For example, CD4+ T cells defend against certain pathogens, including Chlamydia and Leishmania, by starving the pathogens of tryptophan. In those cases, tryptophan starvation works well, since those pathogens are natural tryptophan auxotrophs (lack the ability to synthesize tryptophan). Mycobacterium tuberculosis, on the other hand, is capable of synthesizing tryptophan, and it is protected from this mechanism of host defense; a small molecule inhibitor of M. tuberculosis tryptophan synthesis turns M. tuberculosis into an auxotroph and restores the efficacy of host-mediated tryptophan depletion [119]. Perhaps just as important, even probiotic species such as Lactobacillus spp. are capable of tryptophan biosynthesis and metabolism and generate tryptophan metabolites that activate the aryl hydrocarbon receptor (Ahr), resulting in immunoregulation and mucosal protection from damage [120]. Immunoregulatory bacterially-derived tryptophan metabolites that serve as Ahr agonists include tryptamine, indol-3-acetaldehyde, indole-3-acetic acid, indole-3-aldehyde, and kynurenine [120]. Other bacterially derived tryptophan metabolites that interact with Ahr include indole, 3-methyl-indole, indoxyl sulfate, 6-formylindolo[3,2b] carbazole, and kynurenic acid [121]. Activation of Ahr can either induce functional Treg cells that suppress inflammation, or enhance Th17 cell differentiation and increase inflammation, depending on the specific nature of the agonist [122;123]. Specifically, Ahr induces RORγt+ Tregs [124], consistent with studies discussed above demonstrating that specific species within the gut microbiota can induce RORγt+ Tregs and mucosal immune tolerance [58;59]. Thus, the microbiota, acting via synthesis of tryptophan and generation of tryptophan metabolites that interact with Ahr, regulates both Treg and Th17 cell differentiation in a ligand-specific fashion, constituting a unique target for therapeutic immunomodulation.
Short chain fatty acids (SCFAs)
Short chain fatty acids (SCFAs), including acetate, propionate, and butyrate, are produced by bacteria in the gut during fermentation of insoluble fiber from dietary plant matter [125]. SCFAs support the growth of probiotic Bifidobacterium and Lactobacillus species. Furthermore, SCFAs have antiinflammatory effects by regulating the release of cytokines and chemokines from immune cells [126-128]. Of particular importance, propionate and acetate can directly induce colonic Tregs and their suppressive capacity via activation of G protein coupled receptor (GPCR) 43, encoded by the free fatty acid receptor 2 gene (Ffar2) [129;130]. This is one of the primary routes by which Clostridia affect Treg populations [131]. Furthermore, butyrate, which is also a metabolite of Clostridia species, potentiates DCs to induce Treg through inhibition of histone deacetylase (HDAC) and may induce epigenetic changes [131].
Microbial molecules that induce immunoregulatory responses
A number of bacterial antigens have been identified that increase immunoregulatory circuits, predominantly by interactions with the pattern recognition receptor (PRR) DC-SIGN on DCs. DC-SIGN ligation interferes with toll-like receptor-mediated inflammatory responses, resulting in decreases in nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, decreases in IL-6, TNF, and IL-12, concurrently with increases in IL-10 [101]. Activation of DC-SIGN in DCs may be a common mechanism through which bacterial antigens derived from the old friends bias T cell differentiation toward a Treg phenotype (Table 1). Examples of such molecules, together with their sources, receptors and mode of action are listed in Table 2.
Table 2. Microbial molecules that induced immunoregulatory responses.
| Molecule | Source | Receptor | Actions |
|---|---|---|---|
| Polysaccharide A (PSA) |
Bacteroides
fragilis |
Unclear in mice, DC-SIGN on human DC [194] |
Expands Treg population [195] |
| Surface layer protein A (SlpA) |
Lactobacillus
acidophilus |
DC-SIGN | More IL-10 and less IL-12 p70 [196] |
| Mannose-capped lipoarabinomannan |
Mycobacterium
tuberculosis |
mannose receptor (MR), dectin-2, DC- SIGN, TLR2 [101;197;198] |
Expansion of Treg through a prostaglandin E2-dependent mechanism [199] |
| 60 kDa chaperonin-1 (Cpn60.1); Heat shock 60 kDa protein (Hsp60) |
M. tuberculosis & homologous molecules from microbiota [200] |
DC-SIGN, TLR2 and others? |
Expand Treg [201-204] |
| DnaK; also known as Hsp70, GroP, GrpF, Seg |
M. tuberculosis & homologous molecules from microbiota |
DC-SIGN and others? [200] |
Drive immunoregulatory pathways? [204] |
| Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) |
Mycobacteria and homologous molecules from microbiota [205] |
DC-SIGN [200] | Reduce proinflammatory cytokines and chemokines [206] |
| Lipoarabinomanna n carrier protein LprG |
M. tuberculosis | DC-SIGN [200] | Binds to triacylated glycolipids and increases their agonist activity at TLR2 [101;207] |
| Lewis-X+ lipopolysaccharide (LPS) |
Helicobacter
pylori |
DC-SIGN [103;104] |
Drives Treg responses [98]. |
| Lewis-X+ soluble egg antigen (SEA) |
Schistosoma
mansoni |
DC-SIGN [137] | Drive expansion of Th2 and Treg responses [103;132] |
Other immunoregulatory molecules from “Old Infections”
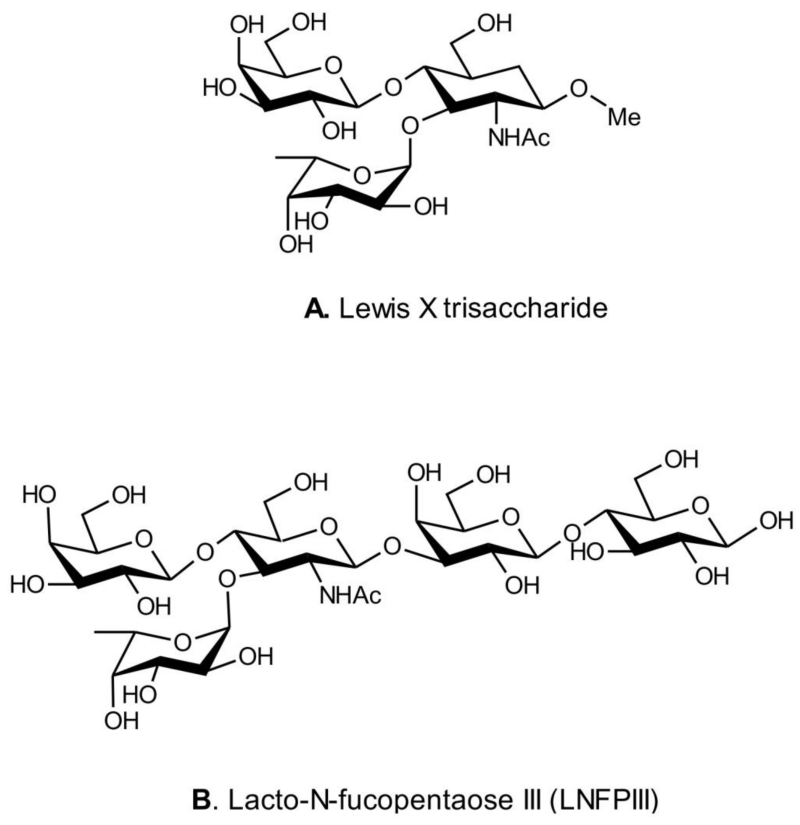
In addition to the heterogeneous group of molecules described above there is mounting interest in microbial products that imitate the LewisX trisaccharide motif (Figure 1).
Figure 1.
Structures of the LewisX trisaccharide motif and its helminth-derived analogue lacto-n-fucopentaose III (LNFPIII).
Lewis-X+lipopolysaccharide (LPS)
The lipopolysaccharide (LPS) Lewis (Le) antigens of H. pylori are able to bind DC-SIGN in gastric DCs and block Th1 development [103;104]. Conversely, Le− H. pylori induce strong Th1 responses [103;104]. Although the specific mechanisms are not clear, H. pylori potently drives Treg responses [98].
Helminthic parasite antigens
The Lewis-X+ soluble egg antigen (SEA) from the helminthic parasite Shistosoma mansoni binds DC-SIGN and induces expansion of Th2 and Treg responses [103;132]. Sole Th2 antiinflammatory immunomodulators apparently emerged when metazoan parasites invaded vertebrates. Indeed, Th2 immunity may have been developed as a repair response to tissue and organ damage resulting from inflammatory processes directed towards relatively large parasites like helminths [133]. Hence, to prevent an inflammatory Th1 immunity, parasitic helminths produced compounds that mimic those that the host makes to avert inflammatory responses that would interfere with tissue healing or cause fetal rejection in mammals; agents that besides inducing Th2, inhibit, but do not abrogate, Th1 immunity [134]. The main family of helminth-derived immunomodulators contains as a pharmacophore the sugar fucose (Fuc); these immunomodulators, which are glycans, structurally and functionally imitate the LewisX trisaccharide motif, like lacto-N-fucopentaose III (LNFPIII), a LewisX-containing immunomodulatory glycan found in human milk [135;136] and SEA from S. mansoni (Fig. 1 A,B). The sugar Fuc is rare in both vertebrates and bacteria and is involved in ontogenesis and some immunological functions, like acting as a Th2 immune modulator. The success of the immunological mimicry developed by helminths is shown by the long-term relationship between the parasite and its host. Fucosylated glycans exert their sole Th2 immune modulatory effects by binding to DC-SIGN, which binds oligosaccharides carrying either mannosyl or fucosyl residues [137]. Any inflammation induced by parasite infection must be tightly regulated and evidence suggests that this function is mediated by induction of Treg by diverse parasites [138].
Membranous cells (M cells) transfer intact gut microorganisms from the bronchopulmonary and intestinal lumens and present them to antigen presenting cells
In order for bacterial antigens, such as the glycans described above, to influence DC function and induce immunoregulatory responses, it is necessary for the bacteria to have direct contact with antigen presenting cells, raising the question of how bacteria in the airways or gastrointestinal tract come into direct contact with these cells. Antigen presenting cells, e.g. alveolar macrophages and CD11c+ DCs, are common in the airways [139], but host mechanisms also ensure that microorganisms in both the airways and gastrointestinal tract can be transferred from the airway or gastrointestinal tract lumen into the body for presentation to antigen-presenting cells. This function is performed in part by microfold or membranous cells (M cells), which are found in both the airways and gastrointestinal tract. M cells in the airways have been documented as entry sites for M. tuberculosis in mice [140]. Similar reports have been made for M cell recognition of mycobacteria in the gastrointestinal tract. Regarding studies involving oral inoculation with mycobacteria, Fujimura wrote that in “1-hour post-inoculated specimens, bacteria were found adhering specifically to M cells, and the microfolds of the M cells were seen to stretch like tentacles toward the bacteria and to catch them.” [141]. Mycobacteria are translocated into M cells, and are subsequently presented to mucosal macrophages and DCs [141]. Although we have focused primarily on the mucosal membranes of the airways and gastrointestinal tract, evidence suggests an important dialogue between the skin microbiota and immune function as well, which should be explored further [142-144].
Antiinflammatory and immunoregulatory interventions
Anti-inflammatory agents such as inhibitors of cycloxygenase, or a neutralizing antibody to TNF are significantly therapeutic in those patients who have raised inflammatory biomarkers [10;11]. Can we therefore exploit the anti-inflammatory properties of immunoregulation-inducing microorganisms? Extensive investigation of animal models has shown that administration of immunoregulatory probiotics and some other manipulations of the microbiota have profound effects on the development and function of the brain. This work has been reviewed recently and will not be described here [145;146], though examples where microbial agents were used in therapeutic models are given in Table 1. Human studies are rare, but there is direct evidence that large doses of probiotics can modulate the human brain. Women were given a milk product fermented with a complex mixture of probiotics twice daily for 4 weeks [147]. Magnetic resonance imaging was used before and after the intervention to measure resting brain activity and the brain response to images of emotional faces. Compared to subjects who had not taken the probiotic, changes in the intrinsic activity of resting brain and in the emotional response were demonstrated [147]. In another randomized, double-blind, placebo-controlled study it was found that when normal volunteers consumed a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) for 30 days, anxiolytic effects were detected by several validated questionnaires [148]. In a very recent study, Clostridium butyricum or placebo were administered orally every morning and evening from 2 weeks before laryngectomy. Hamilton anxiety scores of placebo recipients increased as the surgery approached, but these scores decreased in those taking the probiotic [149]. Finally, recent studies in humans demonstrate that administration of the immunoregulatory probiotic, Lactobacillus casei, for 8 weeks reduced gastrointestinal symptoms and decreased perceived stress in medical students preparing for a standardized exam [150].
However, as we make clear in this review, orally ingested probiotics are not the only possible approach. An environmental soil saprophyte already known to induce Treg in mice [24] was tried as an immunotherapeutic in a human cancer trial [151]. While there was no prolongation of survival it was noted that immunotherapy recipients had improved cognitive functioning and emotional health [151]. More recently the same organism, administered subcutaneously as a killed preparation, has been tested in an animal model where exposure to chronic psychosocial stress induces expansion of Helicobacter spp. [152], pathobionts, that are known to induce colitis in individuals with inadequate immunoregulation [153;154]. Pre-immunisation with the environmental saprophyte blocked stress-induced colitis, blocked stress-induced exaggeration of chemically-induced colitis, and reduced anxiety-related behaviors in stressed mice. Remarkably, these effects were dependent upon the induction of Treg [152].
Conclusions
Exaggerated inflammation is emerging as a risk factor for neurodevelopmental and neuropsychiatric disorders, such as ASD and schizophrenia, and stress-related psychiatric disorders, including PTSD and major depression. Antiinflammatory interventions have shown some promise in treating these conditions. Meanwhile, there is increasing evidence that the microbiota associated with mucosal membranes in the airways and gastrointestinal tract have potential for antiinflammatory and long-term immunoregulatory effects on the host immune system. Thus, bioimmunomodulatory approaches hold promise for the prevention and treatment of these disorders. Here we have highlighted the potential mechanisms through which the microbiota, including microbial inputs from the environment, may elicit antiinflammatory and immunoregulatory responses. The evidence suggests that both bacterial metabolites and bacterial and helminthic antigens have potential for antiinflammatory and immunoregulatory effects that may have value in the prevention and/or treatment of neurodevelopmental, neuropsychiatric, and stress-related mental health disorders.
Acknowledgments
Effort undertaken during the preparation of this review was sponsored by the Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative (MURI) Award, Award No. N00014-15-1-2809.
Footnotes
Conflict of Interest
Christopher A. Lowry, David G. Smith, Philip H. Siebler, Dominic Schmidt, Christopher E. Stamper, James E. Hassell Jr., Paula S. Yamashita, James H. Fox, Stefan O. Reber, Lisa A. Brenner, Andrew J. Hoisington, Teodor T. Postolache, Kerry A. Kinney, Dante Marciani, Mark Hernandez, Sian M.J. Hemmings, Stefanie Malan-Muller, Kenneth P. Wright, Rob Knight, Charles L. Raison, and Graham A.W. Rook report no conflicts of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of outstanding importance
- 1•.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev.Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. This study outlines current evidence for inflammation in the etiology and pathophysiology of depression, and the rationale for antiinflammatory and immunoregulatory approaches to treatment of depression.
- 2.Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, Dean OM, Nardin P, Goncalves CA, Berk M. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol.Psychiatry. 2016;21:554–564. doi: 10.1038/mp.2015.87. [DOI] [PubMed] [Google Scholar]
- 3.Maes M, Ombelet W, Libbrecht I I, Stevens K, Kenis G, De JR, Lin AH, Cox J, Bosmans E. Effects of pregnancy and delivery on serum concentrations of Clara Cell Protein (CC16), an endogenous anticytokine: lower serum CC16 is related to postpartum depression. Psychiatry Res. 1999;87:117–127. doi: 10.1016/s0165-1781(99)00073-6. [DOI] [PubMed] [Google Scholar]
- 4.Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav.Immun. 2013;31:31–47. doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol.Psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rook GA, Lowry CA, Raison CL. Hygiene and other early childhood influences on the subsequent function of the immune system. Brain Res. 2014;1617:47–62. doi: 10.1016/j.brainres.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de P V, Howes OD. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am J Psychiatry. 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 10•.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. This study shows that treatment with an antibody targeting TNF can be beneficial in a subset of depressed patients with increased inflammation.
- 11.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 12.Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane VA, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol. 2015;25:1532–1543. doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, Gilmore JH, Coe CL. Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav.Brain Res. 2011;219:108–115. doi: 10.1016/j.bbr.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J.Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr.Res. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav.Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav.Immun. 2011;25:1272–1278. doi: 10.1016/j.bbi.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryleva EY, Brundin L. Kynurenine pathway metabolites and suicidality. Neuropharmacology. 2016 Jan 26; doi: 10.1016/j.neuropharm.2016.01.034. pii: S0028-3908(16)30033-8. doi: 10.1016/j.neuropharm.2016.01.034. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J.Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin.Immunopathol. 2004;25:237–255. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- 21.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch.Gen.Psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rook GA, Lowry CA. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008;29:150–158. doi: 10.1016/j.it.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Rook GA, Raison CL, Lowry CA. Can we vaccinate against depression? Drug Discov.Today. 2012;17:451–458. doi: 10.1016/j.drudis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Rook GA, Lowry CA, Raison CL. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol.Med Public Health. 2013;2013:46–64. doi: 10.1093/emph/eot004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rook GA, Raison CL, Lowry CA. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol. 2014;177:1–12. doi: 10.1111/cei.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rook GA, Raison CL, Lowry CA. Microbiota, immunoregulatory old friends and psychiatric disorders. Adv.Exp Med Biol. 2014;817:319–356. doi: 10.1007/978-1-4939-0897-4_15. [DOI] [PubMed] [Google Scholar]
- 27.Rook GA, Lowry CA, Raison CL. Hygiene and other early childhood influences on the subsequent function of the immune system. Brain Res. 2015;1617:47–62. doi: 10.1016/j.brainres.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Rook GAW, Lowry CA. The hygiene hypothesis and affective and anxiety disorders. In: Rook GAW, editor. The Hygiene Hypothesis and Darwinian Medicine. Vol. 1. Birkhauser Publishing; Basel: 2009. [Google Scholar]
- 29.Rook GAW, Raison CL, Lowry CA. Childhood microbial experience, immunoregulation, inflammation and adult susceptibility to psychosocial stressors and depression in rich and poor countries. Evolution, Medicine, and Public Health. 2013;2013:14–17. doi: 10.1093/emph/eos005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rook GAW, Raison CL, Lowry CA. In: Microbial “Old Friends”, immunoregulation and psychiatric disorders. Heidt PJ, Midtvedt T, Rusch V, Versalovic J, editors. Vol. 26. Old Herborn University Press; 2013. pp. 61–90. Old Herborn University Monograph. [Google Scholar]
- 31.Hoisington AJ, Brenner LA, Kinney KA, Postolache TT, Lowry CA. The microbiome of the built environment and mental health. Microbiome. 2015;3:1–12. doi: 10.1186/s40168-015-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part I - autointoxication revisited. Gut Pathog. 2013;5:5. doi: 10.1186/1757-4749-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part III - convergence toward clinical trials. Gut Pathog. 2013;5:4. doi: 10.1186/1757-4749-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part II - contemporary contextual research. Gut Pathog. 2013;5:3. doi: 10.1186/1757-4749-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logan AC. Dysbiotic drift: mental health, environmental grey space, and microbiota. J Physiol Anthropol. 2015;34:23. doi: 10.1186/s40101-015-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logan AC, Katzman MA, Balanza-Martinez V. Natural environments, ancestral diets, and microbial ecology: is there a modern “paleo-deficit disorder”? Part II. J Physiol Anthropol. 2015;34:9. doi: 10.1186/s40101-014-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logan AC, Jacka FN, Craig JM, Prescott SL. The microbiome and mental health: Looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol.Neurosci. 2016;14:131–147. doi: 10.9758/cpn.2016.14.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Williamson LL, McKenney EA, Holzknecht ZE, Belliveau C, Rawls JF, Poulton S, Parker W, Bilbo SD. Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav.Immun. 2016;51:14–28. doi: 10.1016/j.bbi.2015.07.006. This study demonstrates that exposure of breeding dams and their offspring with helminths prevents microglial sensitization and cognitive dysfunction in offspring following early life infection with E. coli.
- 39.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55(Suppl 1):S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blaiss MS. Allergic rhinoconjunctivitis: burden of disease. Allergy Asthma Proc. 2007;28:393–397. doi: 10.2500/aap.2007.28.3013. [DOI] [PubMed] [Google Scholar]
- 42.Blaiss MS. Pediatric allergic rhinitis: physical and mental complications. Allergy Asthma Proc. 2008;29:1–6. doi: 10.2500/aap2008.29.3072. [DOI] [PubMed] [Google Scholar]
- 43.Buske-Kirschbaum A, Ebrecht M, Kern S, Gierens A, Hellhammer DH. Personality characteristics in chronic and non-chronic allergic conditions. Brain Behav.Immun. 2008;22:762–768. doi: 10.1016/j.bbi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008;70:102–116. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- 45.Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9. doi: 10.2500/aap.2007.28.2934. [DOI] [PubMed] [Google Scholar]
- 46.Wright RJ. Stress and atopic disorders. J Allergy Clin Immunol. 2005;116:1301–1306. doi: 10.1016/j.jaci.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin RD, Fergusson DM, Horwood LJ. Asthma and depressive and anxiety disorders among young persons in the community. Psychol Med. 2004;34:1465–1474. doi: 10.1017/s0033291704002739. [DOI] [PubMed] [Google Scholar]
- 48.Goodwin RD. Self-reported hay fever and panic attacks in the community. Ann.Allergy Asthma Immunol. 2002;88:556–559. doi: 10.1016/S1081-1206(10)61885-6. [DOI] [PubMed] [Google Scholar]
- 49.Palermo-Neto J, Guimaraes RK. Pavlovian conditioning of lung anaphylactic response in rats. Life Sci. 2000;68:611–623. doi: 10.1016/s0024-3205(00)00966-8. [DOI] [PubMed] [Google Scholar]
- 50.Tonelli LH, Katz M, Kovacsics CE, Gould TD, Joppy B, Hoshino A, Hoffman G, Komarow H, Postolache TT. Allergic rhinitis induces anxiety-like behavior and altered social interaction in rodents. Brain Behav.Immun. 2009;23:784–793. doi: 10.1016/j.bbi.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. This study shows that IL-17A from the mother mediates development of an autism-like phenotype in a mouse model of maternal infection.
- 52.Mostafa GA, Al SA, Fouad NR. Frequency of CD4+CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. J Child Neurol. 2010;25:328–335. doi: 10.1177/0883073809339393. [DOI] [PubMed] [Google Scholar]
- 53.Al-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. 2012;9:158. doi: 10.1186/1742-2094-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. This study shows that exposure to immunoregulatory bacteria early in development can prevent some aspects of an autism-like phenotype in a mouse model of maternal infection.
- 55.Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 56.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu.Rev.Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, Ghosh S, Earl A, Snapper SB, Jupp R, Kasper D, Mathis D, Benoist C. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;349:993–997. doi: 10.1126/science.aaa9420. This study shows that individual species of immunoregulatory bacteria can restore RORγ+ Treg in germ-free mice.
- 59.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, Busslinger M, Cerf-Bensussan N, Boneca IG, Voehringer D, Hase K, Honda K, Sakaguchi S, Eberl G. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 60.Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev.Neurobiol. 2012;72:1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. J Autism Dev.Disord. 2005;35:471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- 62.Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, Weisskopf MG. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ.Health Perspect. 2015;123:264–270. doi: 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dietert RR, Dietert JM. Potential for early-life immune insult including developmental immunotoxicity in autism and autism spectrum disorders: focus on critical windows of immune vulnerability. J Toxicol.Environ.Health B Crit Rev. 2008;11:660–680. doi: 10.1080/10937400802370923. [DOI] [PubMed] [Google Scholar]
- 64.Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de WC. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Taliou A, Zintzaras E, Lykouras L, Francis K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther. 2013;35:592–602. doi: 10.1016/j.clinthera.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl.Psychiatry. 2015;5:e647. doi: 10.1038/tp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol.Psychiatry. 2013;73:993–999. doi: 10.1016/j.biopsych.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Debnath M, Berk M. Th17 pathway-mediated immunopathogenesis of schizophrenia: mechanisms and implications. Schizophr.Bull. 2014;40:1412–1421. doi: 10.1093/schbul/sbu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sommer IE, de WL, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73:414–419. doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- 70.Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol.Psychiatry. 2014;75:332–341. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Machado CJ, Whitaker AM, Smith SE, Patterson PH, Bauman MD. Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol.Psychiatry. 2015;77:823–832. doi: 10.1016/j.biopsych.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weir RK, Forghany R, Smith SE, Patterson PH, McAllister AK, Schumann CM, Bauman MD. Preliminary evidence of neuropathology in nonhuman primates prenatally exposed to maternal immune activation. Brain Behav.Immun. 2015;48:139–146. doi: 10.1016/j.bbi.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. This study shows that elevated plasma CRP predicts subsequent risk for development of PTSD.
- 74•.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonte B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolanos-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc.Natl.Acad.Sci.U.S.A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. This study demonstrates that individual differences in peripheral immune status, particularly LPS-induced release of IL-6, predicts individual variability in development of anxiety and depressive-like behavioral responses to a subsequent psychosocial stressor in mice.
- 75.Kivimaki M, Shipley MJ, Batty GD, Hamer M, Akbaraly TN, Kumari M, Jokela M, Virtanen M, Lowe GD, Ebmeier KP, Brunner EJ, Singh-Manoux A. Long-term inflammation increases risk of common mental disorder: a cohort study. Mol.Psychiatry. 2014;19:149–150. doi: 10.1038/mp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76:181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 77.Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–999. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Spitzer C, Barnow S, Volzke H, Wallaschofski H, John U, Freyberger HJ, Lowe B, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr.Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR, Hobfoll SE. Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine. 2013;63:172–178. doi: 10.1016/j.cyto.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 81.Andrews JA, Neises KD. Cells, biomarkers, and post-traumatic stress disorder: evidence for peripheral involvement in a central disease. J.Neurochem. 2012;120:26–36. doi: 10.1111/j.1471-4159.2011.07545.x. [DOI] [PubMed] [Google Scholar]
- 82.Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, Kolassa IT. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC.Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, Neylan TC. Elevated Risk for Autoimmune Disorders in Iraq and Afghanistan Veterans with Posttraumatic Stress Disorder. Biol.Psychiatry. 2014;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stein MB, Chen C-Y, Ursano RJ, Cai T, Gelernter J, Heeringa SG, Jain S, Jensen KP, Maihofer AX, Mitchell C, Nievergelt CM, Nock MK, Neale BM, Polimanti R, Ripke S, Sun X, Thomas ML, Wang Q, Ware EB, Borja S, Kessler RC, Smoller JW, for the Army Study to Assess Risk and Resilience in Servicemembers (STARRS) Collaborators Genome-wide association studies of posttraumatic stress disorder in 2 cohorts of US Army soldiers. JAMA Psychiatry. 2016:E1–E10. doi: 10.1001/jamapsychiatry.2016.0350. Published online May 11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lill CM, Schjeide BM, Graetz C, Liu T, Damotte V, Akkad DA, Blaschke P, Gerdes LA, Kroner A, Luessi F, Cournu-Rebeix I, Hoffjan S, Winkelmann A, Touze E, Pico F, Corcia P, Otaegui D, Antiguedad A, Alcina A, Comabella M, Montalban X, Olascoaga J, Matesanz F, Dorner T, Li SC, Steinhagen-Thiessen E, Lindenberger U, Chan A, Rieckmann P, Hartung HP, Aktas O, Lohse P, Buttmann M, Kumpfel T, Kubisch C, Zettl UK, Epplen JT, Fontaine B, Zipp F, Vandenbroeck K, Bertram L. Genome-wide significant association of ANKRD55 rs6859219 and multiple sclerosis risk. J Med Genet. 2013;50:140–143. doi: 10.1136/jmedgenet-2012-101411. [DOI] [PubMed] [Google Scholar]
- 86.Alloza I, Otaegui D, de Lapuente AL, Antiguedad A, Varade J, Nunez C, Arroyo R, Urcelay E, Fernandez O, Leyva L, Fedetz M, Izquierdo G, Lucas M, Oliver-Martos B, Alcina A, Saiz A, Blanco Y, Comabella M, Montalban X, Olascoaga J, Matesanz F, Vandenbroeck K. ANKRD55 and DHCR7 are novel multiple sclerosis risk loci. Genes Immun. 2012;13:253–257. doi: 10.1038/gene.2011.81. [DOI] [PubMed] [Google Scholar]
- 87.Harder MN, Ribel-Madsen R, Justesen JM, Sparso T, Andersson EA, Grarup N, Jorgensen T, Linneberg A, Hansen T, Pedersen O. Type 2 diabetes risk alleles near BCAR1 and in ANK1 associate with decreased beta-cell function whereas risk alleles near ANKRD55 and GRB14 associate with decreased insulin sensitivity in the Danish Inter99 cohort. J Clin Endocrinol.Metab. 2013;98:E801–E806. doi: 10.1210/jc.2012-4169. [DOI] [PubMed] [Google Scholar]
- 88.Zhernakova A, Stahl EA, Trynka G, Raychaudhuri S, Festen EA, Franke L, Westra HJ, Fehrmann RS, Kurreeman FA, Thomson B, Gupta N, Romanos J, McManus R, Ryan AW, Turner G, Brouwer E, Posthumus MD, Remmers EF, Tucci F, Toes R, Grandone E, Mazzilli MC, Rybak A, Cukrowska B, Coenen MJ, Radstake TR, van Riel PL, Li Y, de Bakker PI, Gregersen PK, Worthington J, Siminovitch KA, Klareskog L, Huizinga TW, Wijmenga C, Plenge RM. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS.Genet. 2011;7:e1002004. doi: 10.1371/journal.pgen.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viatte S, Plant D, Bowes J, Lunt M, Eyre S, Barton A, Worthington J. Genetic markers of rheumatoid arthritis susceptibility in anti-citrullinated peptide antibody negative patients. Ann Rheum.Dis. 2012;71:1984–1990. doi: 10.1136/annrheumdis-2011-201225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De VR, Steptoe A, Kumari M, Lowe GD, Rumley A, Marmot MG, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol.Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amos T, Stein DJ, Ipser JC. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD) Cochrane.Database.Syst.Rev. 2014;7:CD006239. doi: 10.1002/14651858.CD006239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Na KS, Lee KJ, Lee JS, Cho YS, Jung HY. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2014;48:79–85. doi: 10.1016/j.pnpbp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Martinez I, Stegen JC, Maldonado-Gomez MX, Eren AM, Siba PM, Greenhill AR, Walter J. The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep. 2015;11:527–538. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 95.Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, Magris M, Hidalgo G, Contreras M, Noya-Alarcon O, Lander O, McDonald J, Cox M, Walter J, Oh PL, Ruiz JF, Rodriguez S, Shen N, Song SJ, Metcalf J, Knight R, Dantas G, Dominguez-Bello MG. The microbiome of uncontacted Amerindians. Sci.Adv. 2015 Apr 17;1:e1500183, 1–12. doi: 10.1126/sciadv.1500183. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maixner F, Krause-Kyora B, Turaev D, Herbig A, Hoopmann MR, Hallows JL, Kusebauch U, Vigl EE, Malfertheiner P, Megraud F, O’Sullivan N, Cipollini G, Coia V, Samadelli M, Engstrand L, Linz B, Moritz RL, Grimm R, Krause J, Nebel A, Moodley Y, Rattei T, Zink A. The 5300-year-old Helicobacter pylori genome of the Iceman. Science. 2016;351:162–165. doi: 10.1126/science.aad2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, Muller A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arnold IC, Hitzler I, Muller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect.Microbiol. 2012;2:10. doi: 10.3389/fcimb.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rook GAW, Raison CL, Lowry CA. Microbes and mood: a new approach to the therapy of depression? Microbiologist. 2011 Sep;:32–36. [Google Scholar]
- 100.Gillespie L, Roosendahl P, Ng WC, Brooks AG, Reading PC, Londrigan SL. Endocytic function is critical for influenza A virus infection via DC-SIGN and L-SIGN. Sci.Rep. 2016;6:19428. doi: 10.1038/srep19428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vergne I, Gilleron M, Nigou J. Manipulation of the endocytic pathway and phagocyte functions by Mycobacterium tuberculosis lipoarabinomannan. Front Cell Infect.Microbiol. 2014;4:187. doi: 10.3389/fcimb.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smits HH, Hartgers FC, Yazdanbakhsh M. Helminth infections: protection from atopic disorders. Curr.Allergy Asthma Rep. 2005;5:42–50. doi: 10.1007/s11882-005-0053-5. [DOI] [PubMed] [Google Scholar]
- 103.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat.Rev.Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 104.Bergman MP, Engering A, Smits HH, van Vliet SJ, van Bodegraven AA, Wirth HP, Kapsenberg ML, Vandenbroucke-Grauls CM, van KY, Appelmelk BJ. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Appelmelk BJ, van D I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, van KY. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 106.Hunt JR, Martinelli R, Adams VC, Rook GA, Brunet LR. Intragastric administration of Mycobacterium vaccae inhibits severe pulmonary allergic inflammation in a mouse model. Clin.Exp.Allergy. 2005;35:685–690. doi: 10.1111/j.1365-2222.2005.02239.x. [DOI] [PubMed] [Google Scholar]
- 107.Moore MN. Do airborne biogenic chemicals interact with the PI3K/Akt/mTOR cell signalling pathway to benefit human health and wellbeing in rural and coastal environments? Environ.Res. 2015;140:65–75. doi: 10.1016/j.envres.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 108.Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc.Natl.Acad.Sci.U.S.A. 2013;110:18360–18367. doi: 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rook GAW. Introduction: The changing microbial environment, Darwinian medicine and the hygiene hypothesis. In: Rook GAW, editor. The Hygiene Hypothesis and Darwinian Medicine. Birkhäuser; Basel: 2009. pp. 1–27. [Google Scholar]
- 110.Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat.Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 111.Holinger EP, Ross KA, Robertson CE, Stevens MJ, Harris JK, Pace NR. Molecular analysis of point-of-use municipal drinking water microbiology. Water Res. 2014;49:225–235. doi: 10.1016/j.watres.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 112.Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc.Natl.Acad.Sci.U.S.A. 2009;106:16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113•.Macovei L, McCafferty J, Chen T, Teles F, Hasturk H, Paster BJ, Campos-Neto A. The hidden ‘mycobacteriome’ of the human healthy oral cavity and upper respiratory tract. J Oral Microbiol. 2015;7:26094. doi: 10.3402/jom.v7.26094. This study documents abundant environmental microbes in the mucosa of the oral cavity and upper airways of healthy human volunteers.
- 114.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc.Natl.Acad.Sci.U.S.A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J.Exp.Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nowak EC, de Vries VC, Wasiuk A, Ahonen C, Bennett KA, Le M I, Ha DG, Noelle RJ. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J Exp Med. 2012;209:2127–2135. doi: 10.1084/jem.20120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farez MF, Mascanfroni ID, Mendez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, Balbuena Aguirre ME, Patel B, Ysrraelit MC, Zhu C, Kuchroo VK, Rabinovich GA, Quintana FJ, Correale J. Melatonin Contributes to the Seasonality of Multiple Sclerosis Relapses. Cell. 2015;162:1338–1352. doi: 10.1016/j.cell.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang YJ, Reddy MC, Ioerger TR, Rothchild AC, Dartois V, Schuster BM, Trauner A, Wallis D, Galaviz S, Huttenhower C, Sacchettini JC, Behar SM, Rubin EJ. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell. 2013;155:1296–1308. doi: 10.1016/j.cell.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zelante T, Iannitti RG, Cunha C, De LA, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 121.Zhang L, Nichols RG, Correll J, Murray IA, Tanaka N, Smith PB, Hubbard TD, Sebastian A, Albert I, Hatzakis E, Gonzalez FJ, Perdew GH, Patterson AD. Persistent Organic Pollutants Modify Gut Microbiota-Host Metabolic Homeostasis in Mice Through Aryl Hydrocarbon Receptor Activation. Environ.Health Perspect. 2015;123:679–688. doi: 10.1289/ehp.1409055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 123.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qiu J, Zhou L. Aryl hydrocarbon receptor promotes RORgammat(+) group 3 ILCs and controls intestinal immunity and inflammation. Semin.Immunopathol. 2013;35:657–670. doi: 10.1007/s00281-013-0393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20:779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cavaglieri CR, Nishiyama A, Fernandes LC, Curi R, Miles EA, Calder PC. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–1690. doi: 10.1016/s0024-3205(03)00490-9. [DOI] [PubMed] [Google Scholar]
- 127.Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meijer K, de VP, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr.Opin.Clin Nutr.Metab Care. 2010;13:715–721. doi: 10.1097/MCO.0b013e32833eebe5. [DOI] [PubMed] [Google Scholar]
- 129.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly Y, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arpaia N, Campbell C, Fan X, Dikiy S, van d V, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015;36:3–12. doi: 10.1016/j.it.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39:1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 133.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev.Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y, Da’Dara AA, Thomas PG, Harn DA. Dendritic cells activated by an anti-inflammatory agent induce CD4(+) T helper type 2 responses without impairing CD8(+) memory and effector cytotoxic T-lymphocyte responses. Immunology. 2010;129:406–417. doi: 10.1111/j.1365-2567.2009.03193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stahl B, Thurl S, Zeng J, Karas M, Hillenkamp F, Steup M, Sawatzki G. Oligosaccharides from human milk as revealed by matrix-assisted laser desorption/ionization mass spectrometry. Anal.Biochem. 1994;223:218–226. doi: 10.1006/abio.1994.1577. [DOI] [PubMed] [Google Scholar]
- 136.Ko AI, Drager UC, Harn DA. A Schistosoma mansoni epitope recognized by a protective monoclonal antibody is identical to the stage-specific embryonic antigen 1. Proc.Natl.Acad.Sci.U.S.A. 1990;87:4159–4163. doi: 10.1073/pnas.87.11.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Liempt E, Bank CM, Mehta P, Garcia-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van D I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 138.Adalid-Peralta L, Fragoso G, Fleury A, Sciutto E. Mechanisms Underlying the Induction of Regulatory T cells and Its Relevance in the Adaptive Immune Response in Parasitic Infections. International Journal of Biological Sciences. 2011;7:1412–1426. doi: 10.7150/ijbs.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev.Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 140.Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, Pollard JW, McMurray DN, Bloom BR. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10:641–650. doi: 10.1016/s1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- 141.Fujimura Y. Functional morphology of microfold cells (M cells) in Peyer’s patches--phagocytosis and transport of BCG by M cells into rabbit Peyer’s patches. Gastroenterol Jpn. 1986;21:325–335. [PubMed] [Google Scholar]
- 142.Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–959. doi: 10.1126/science.1260144. [DOI] [PubMed] [Google Scholar]
- 143.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol. 2013;14:646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145••.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat.Rev.Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. This article provides a comprehensive review on the microbiota-gut-brain and behavior axis.
- 146.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J.Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147••.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–401. 1401. doi: 10.1053/j.gastro.2013.02.043. This study provides the first evidence that probiotic administration in healthy humans modifies steady state activity of neural systems controlling cognitive and affective function.
- 148.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br.J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 149.Yang H, Zhao X, Tang S, Huang H, Zhao X, Ning Z, Fu X, Zhang C. Probiotics reduce psychological stress in patients before laryngeal cancer surgery. Asia Pac.J Clin Oncol. 2016;12:e92–e96. doi: 10.1111/ajco.12120. [DOI] [PubMed] [Google Scholar]
- 150.Kato-Kataoka A, Nishida K, Takada M, Suda K, Kawai M, Shimizu K, Kushiro A, Hoshi R, Watanabe O, Igarashi T, Miyazaki K, Kuwano Y, Rokutan K. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef.Microbes. 2016;7:153–156. doi: 10.3920/BM2015.0100. [DOI] [PubMed] [Google Scholar]
- 151.O’Brien ME, Anderson H, Kaukel E, O’Byrne K, Pawlicki M, von Pawel J, Reck M. SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: phase III results. Ann.Oncol. 2004;15:906–914. doi: 10.1093/annonc/mdh220. [DOI] [PubMed] [Google Scholar]
- 152•.Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, Lowe KR, Campbell K, Fox JH, Hassell JEJ, Greenwood BN, Jansch C, Lechner A, Schmidt D, Uschold-Schmidt N, Füchsl AM, Langgartner D, Walker FR, Hale MW, Lopez Perez G, Van Treuren W, González A, Halweg-Edwards AL, Fleshner M, Raison CL, Rook GA, Peddada SD, Knight R, Lowry CA. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc.Natl.Acad.Sci.U.S.A. 2016 May 16; doi: 10.1073/pnas.1600324113. 2016. pii: 201600324. [Epub ahead of print] This study provides the first evidence that immunization with a heat-killed preparation of an environmental immunoregulatory microbe can prevent colitis and reduce anxiety-related behaviors in mice.
- 153.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect.Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, Viney JL, Maggio-Price L. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest.Liver Physiol. 2001;281:G764–G778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- 155.Collins CH, Grange JM, Yates MD. Mycobacteria in water. J Appl.Bacteriol. 1984;57:193–211. doi: 10.1111/j.1365-2672.1984.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 156.Stanford JL, Paul RC. A preliminary report on some studies of environmental mycobacteria from Uganda. Ann.Soc Belg.Med Trop. 1973;53:389–393. [PubMed] [Google Scholar]
- 157.Primm TP, Lucero CA, Falkinham JO., III Health impacts of environmental mycobacteria. Clin Microbiol.Rev. 2004;17:98–106. doi: 10.1128/CMR.17.1.98-106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jin BW, Saito H, Yoshii Z. Environmental mycobacteria in Korea. I. Distribution of the organisms. Microbiol.Immunol. 1984;28:667–677. doi: 10.1111/j.1348-0421.1984.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 159.Pontiroli A, Khera TT, Oakley BB, Mason S, Dowd SE, Travis ER, Erenso G, Aseffa A, Courtenay O, Wellington EM. Prospecting environmental mycobacteria: combined molecular approaches reveal unprecedented diversity. PLoS.ONE. 2013;8:e68648. doi: 10.1371/journal.pone.0068648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gcebe N, Rutten V, van Pittius NC, Michel A. Prevalence and distribution of non-tuberculous mycobacteria (NTM) in cattle, African buffaloes (Syncerus caffer) and their environments in South Africa. Transbound.Emerg.Dis. 2013;60(Suppl 1):74–84. doi: 10.1111/tbed.12133. [DOI] [PubMed] [Google Scholar]
- 161.Kamala T, Paramasivan CN, Herbert D, Venkatesan P, Prabhakar R. Isolation and Identification of Environmental Mycobacteria in the Mycobacterium bovis BCG Trial Area of South India. Appl.Environ.Microbiol. 1994;60:2180–2183. doi: 10.1128/aem.60.6.2180-2183.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Le Bert N, Chain BM, Rook G, Noursadeghi M. DC priming by M. vaccae inhibits Th2 responses in contrast to specific TLR2 priming and is associated with selective activation of the CREB pathway. PLoS.ONE. 2011;6:e18346. doi: 10.1371/journal.pone.0018346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lowry CA, Hollis JH, de VA, Pan B, Brunet LR, Hunt JR, Paton JF, van KE, Knight DM, Evans AK, Rook GA, Lightman SL. Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior. Neuroscience. 2007;146:756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matthews DM, Jenks SM. Ingestion of Mycobacterium vaccae decreases anxiety-related behavior and improves learning in mice. Behav.Processes. 2013;96:27–35. doi: 10.1016/j.beproc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 165.Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav.Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 166.Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol.Motil. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 167.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 168.Zuo L, Yuan KT, Yu L, Meng QH, Chung PC, Yang DH. Bifidobacterium infantis attenuates colitis by regulating T cell subset responses. World J Gastroenterol. 2014;20:18316–18329. doi: 10.3748/wjg.v20.i48.18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 170.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol.Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 172.Lopez P, Gonzalez-Rodriguez I, Gueimonde M, Margolles A, Suarez A. Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLoS.ONE. 2011;6:e24776. doi: 10.1371/journal.pone.0024776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Castillo-Rojas G, Mazari-Hiriart M, Ponce de LS, Amieva-Fernandez RI, Agis-Juarez RA, Huebner J, Lopez-Vidal Y. Comparison of Enterococcus faecium and Enterococcus faecalis Strains isolated from water and clinical samples: antimicrobial susceptibility and genetic relationships. PLoS.ONE. 2013;8:e59491. doi: 10.1371/journal.pone.0059491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Divyashri G, Krishna G, Muralidhara M, Prapulla SG. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of probiotic Enterococcus faecium CFR 3003: In vitro and in vivo evidences. J Med Microbiol. 2015;64:1527–1540. doi: 10.1099/jmm.0.000184. [DOI] [PubMed] [Google Scholar]
- 176.Bernardeau M, Guguen M, Vernoux JP. Beneficial lactobacilli in food and feed: long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol.Rev. 2006;30:487–513. doi: 10.1111/j.1574-6976.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- 177.Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 179.Vitali B, Minervini G, Rizzello CG, Spisni E, Maccaferri S, Brigidi P, Gobbetti M, Di CR. Novel probiotic candidates for humans isolated from raw fruits and vegetables. Food Microbiol. 2012;31:116–125. doi: 10.1016/j.fm.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 180.Wood BJB, Holzapfel WH, editors. The Genera of Lactic Acid Bacteria. Chapman & Hall; 1995. The genus Lactobacillus; pp. 19–54. [Google Scholar]
- 181.Wang T, Hu X, Liang S, Li W, Wu X, Wang L, Jin F. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef.Microbes. 2015:1–11. doi: 10.3920/BM2014.0177. [DOI] [PubMed] [Google Scholar]
- 182.Perez-Cano FJ, Dong H, Yaqoob P. In vitro immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: two probiotic strains isolated from human breast milk. Immunobiology. 2010;215:996–1004. doi: 10.1016/j.imbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 183.Chung YC, Jin HM, Cui Y, Kim DS, Jung JM, Park JI, Jung ES, Choi EK, Chae SW. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. Journal of Functional Foods. 2014;10:465–474. [Google Scholar]
- 184.Jia L, Tao W, Shan L, Xu H, Wei L, Feng J. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Science China Life Sciences. 2014;57:327–335. doi: 10.1007/s11427-014-4615-4. [DOI] [PubMed] [Google Scholar]
- 185.Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 186.Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E, Madsen KL. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38:1738–1747. doi: 10.1016/j.psyneuen.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 187.Smith CJ, Emge JR, Berzins K, Lung L, Khamishon R, Shah P, Rodrigues DM, Sousa AJ, Reardon C, Sherman PM, Barrett KE, Gareau MG. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol Gastrointest.Liver Physiol. 2014;307:G793–G802. doi: 10.1152/ajpgi.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, MacQueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 189.Rodrigues DM, Sousa AJ, Johnson-Henry KC, Sherman PM, Gareau MG. Probiotics are effective for the prevention and treatment of Citrobacter rodentium-induced colitis in mice. J Infect.Dis. 2012;206:99–109. doi: 10.1093/infdis/jis177. [DOI] [PubMed] [Google Scholar]
- 190.Woo JY, Gu W, Kim KA, Jang SE, Han MJ, Kim DH. Lactobacillus pentosus var. plantarum C29 ameliorates memory impairment and inflammaging in a D-galactose-induced accelerated aging mouse model. Anaerobe. 2014;27:22–26. doi: 10.1016/j.anaerobe.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 191.Tubelius P, Stan V, Zachrisson A. Increasing work-place healthiness with the probiotic Lactobacillus reuteri: a randomised, double-blind placebo-controlled study. Environ.Health. 2005;4:25. doi: 10.1186/1476-069X-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van KY, Kapsenberg ML. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 193.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc.Natl.Acad.Sci.U.S.A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bloem K, Garcia-Vallejo JJ, Vuist IM, Cobb BA, van Vliet SJ, van Kooyk Y. Interaction of the Capsular Polysaccharide A from Bacteroides fragilis with DC-SIGN on Human Dendritic Cells is Necessary for Its Processing and Presentation to T Cells. Front Immunol. 2013;4:103. doi: 10.3389/fimmu.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host.Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van KY. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc.Natl.Acad.Sci.U.S.A. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gilleron M, Jackson M, Nigou J, Puzo G. Structure, biosynthesis, and activities of the phosphatidyl-myo-inositol-based lipoglycans. In: Daffé M, Reyrat JM, editors. The Mycobacterial Cell Envelope. ASM Press; Washington, DC: 2008. pp. 75–105. [Google Scholar]
- 198.Ray A, Cot M, Puzo G, Gilleron M, Nigou J. Bacterial cell wall macroamphiphiles: pathogen-/microbe-associated molecular patterns detected by mammalian innate immune system. Biochimie. 2013;95:33–42. doi: 10.1016/j.biochi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 199.Garg A, Barnes PF, Roy S, Quiroga MF, Wu S, Garcia VE, Krutzik SR, Weis SE, Vankayalapati R. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008;38:459–469. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Carroll MV, Sim RB, Bigi F, Jakel A, Antrobus R, Mitchell DA. Identification of four novel DC-SIGN ligands on Mycobacterium bovis BCG. Protein Cell. 2010;1:859–870. doi: 10.1007/s13238-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Quintana FJ, Cohen IR. The HSP60 immune system network. Trends Immunol. 2011;32:89–95. doi: 10.1016/j.it.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 202.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 203.Ohue R, Hashimoto K, Nakamoto M, Furukawa Y, Masuda T, Kitabatake N, Tani F. Bacterial heat shock protein 60, GroEL, can induce the conversion of naive T cells into a CD4 CD25(+) Foxp3-expressing phenotype. J Innate.Immun. 2011;3:605–613. doi: 10.1159/000330786. [DOI] [PubMed] [Google Scholar]
- 204.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev.Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 205.Ercolani L, Florence B, Denaro M, Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol.Chem. 1988;263:15335–15341. [PubMed] [Google Scholar]
- 206.Takaoka Y, Goto S, Nakano T, Tseng HP, Yang SM, Kawamoto S, Ono K, Chen CL. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced, sepsis-related severe acute lung injury in mice. Sci.Rep. 2014;4:5204. doi: 10.1038/srep05204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Drage MG, Tsai HC, Pecora ND, Cheng TY, Arida AR, Shukla S, Rojas RE, Seshadri C, Moody DB, Boom WH, Sacchettini JC, Harding CV. Mycobacterium tuberculosis lipoprotein LprG (Rv1411c) binds triacylated glycolipid agonists of Toll-like receptor 2. Nat Struct.Mol.Biol. 2010;17:1088–1095. doi: 10.1038/nsmb.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]