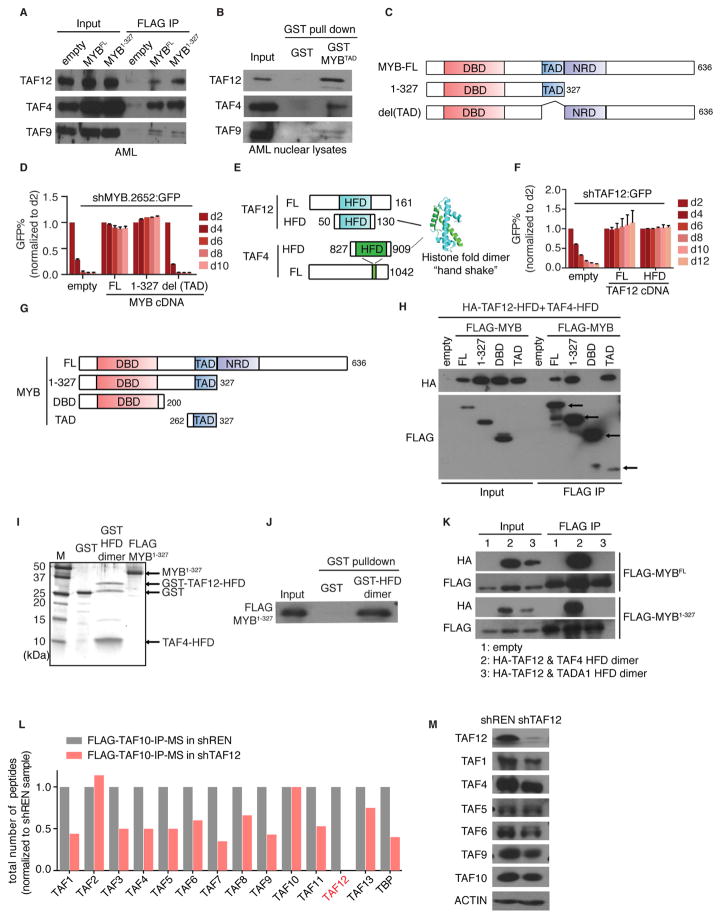
Figure 4. Physical interaction between MYB and the TAF12/TAF4 heterodimer.
(A) IP-western blot analysis evaluating the interaction between FLAG-MYB and endogenous TFIID subunits. FLAG IP was performed in nuclear lysates from RN2 cells stably expressing FLAG tagged MYBFL(full-length), MYB1-327 fragment or empty vector followed by western blotting for the indicated TFIID subunits.
(B) Western blot analysis of TFIID subunits pulled down by recombinant GST or GST tagged transactivation domain (TAD) of MYB from RN2 nuclear lysates.
(C) Illustration of MYB domain architecture and fragments used for shRNA/cDNA rescue experiments. DBD: DNA-binding domain, TAD: transactivation domain, NRD: negative regulatory domain.
(D) GFP depletion assay evaluating the FLAG-MYB fragments shown in (C) to rescue the growth-arrest in RN2 cells caused by GFP-linked MYB shRNA (#2652). Bar graph represents the mean ±3SEM, n=3.
(E) Illustration of murine TAF12 and TAF4 domain architectures. Crystal structure represents the human TAF12/TAF4 histone-fold domain (HFD) heterodimer (PDB:1H3O) (Werten et al., 2002).
(F) GFP depletion assay evaluating the ability of full-length (FL) TAF12 versus the TAF12-HFD fragment (amino acids 50–130) to rescue the growth-arrest in RN2 cells caused by GFP-linked TAF12 shRNA (#364). Bar graph represents the mean ±3SEM, n=3.
(G) Illustration of MYB truncation fragments used for co-IP experiments.
(H) Co-IP western blot analysis evaluating the interaction between the indicated MYB fragments and the TAF4/TAF12 HFD heterodimer. HEK 293T cells were co-transfected with HA tagged TAF12, untagged TAF4-HFD, and one of the FLAG-MYB fragments shown in (G) or empty vector. FLAG IP was performed in nuclear lysates 48 hr post transfection.
(I) Silver staining analysis of the recombinant GST, GST tagged TAF12/TAF4 HFD heterodimer (purified from E. coli) and FLAG-MYB1-327 (purified from HEK 293T).
(J) Binding assay using the proteins shown in (I). GST proteins were immobilized on glutathione beads, followed by incubation with FLAG-MYB1-327. The bead-associated complexes were eluted and western blotted with anti-FLAG antibodies.
(K) Co-IP western blot analysis evaluating the interaction between MYB and TADA1/TAF12 or TAF4/TAF12 HFD heterodimer. HEK 293T cells were co-transfected with HA tagged TAF12-HFD, untagged TAF4-HFD or TADA1-HFD, and FLAG-MYB (FL or 1-327 fragment) or empty vector. FLAG IP was performed in nuclear lysates 48 hr post transfection.
(L) Mass spectrometry analysis of TFIID subunits following FLAG-TAF10 IP in RN2 cells expressing a dox-regulated TAF12 shRNA (#364) or control shRNA. Total peptide counts for the indicated subunits is shown, normalized to the control shRNA samples.
(M) Western blot analysis of the indicated TFIID subunits in RN2 cells on day 3 post infection of control or TAF12 (#364) shRNAs.
See also Figure S4.