Abstract
Neutrophil elastase (NE) is an innate immune cell-derived inflammatory mediator that we have shown increases the presentation of tumor-associated peptide antigens in breast cancer. In this study, we extend these observations to show that NE uptake has a broad effect on enhancing antigen presentation by breast cancer cells. We show that NE increases human leukocyte antigen (HLA) class I expression on the surface of breast cancer cells in a concentration and time-dependent manner. HLA class I upregulation requires internalization of enzymatically active NE. Western blots of NE-treated breast cancer cells confirm that the expression of total HLA class I as well as the antigen-processing machinery proteins TAP1, LMP2, and calnexin does not change following NE treatment. This suggests that NE does not increase the efficiency of antigen processing; rather, it mediates the upregulation of HLA class I by stabilizing and reducing membrane recycling of HLA class I molecules. Furthermore, the effects of NE extend beyond breast cancer since the uptake of NE by EBV–LCL increases the presentation of HLA class I-restricted viral peptides, as shown by their increased sensitivity to lysis by EBV-specific CD8+ T cells. Together, our results show that NE uptake increases the responsiveness of breast cancer cells to adaptive immunity by broad upregulation of membrane HLA class I and support the conclusion that the innate inflammatory mediator NE enhances tumor cell recognition and increases tumor sensitivity to the host adaptive immune response.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1841-6) contains supplementary material, which is available to authorized users.
Keywords: Neutrophil elastase, Tumor-associated neutrophils, HLA class I, Breast cancer, Adaptive immunity
Introduction
Inflammation has been shown to play a role in cancer development and progression. A number of studies have shown beneficial anti-tumor effects of inflammatory cells in controlling tumor growth [1, 2], while other studies have correlated inflammation with negative clinical outcomes [3, 4]. These dichotomous results have been attributed to a number of components of the tumor microenvironment, including tumor-associated neutrophils (TANs) and tumor-associated macrophages (TAMs) that have both pro- and anti-tumor effects on the tumor depending in part on other inflammatory mediators within the tumor milieu. TANs and TAMs within the tumor microenvironment produce azurophilic granule serine proteases, including neutrophil elastase (NE), that have been implicated in modulating tumor behavior both favorably and unfavorably [5–8]. The exact mechanisms have not been elucidated.
NE is a serine protease expressed in myeloid hematopoietic cells. Within the tumor microenvironment, NE is released from storage granules within TANs in response to interleukin 8 and tumor necrosis factor (TNF) alpha, which are increased at sites of inflammation [9, 10]. Although NE has been detected in tumor tissues, including breast cancer, where it was demonstrated to be a poor prognostic factor [6, 11], a number of more recent studies, including our own, have shown that TAN and soluble innate immune mediators derived from TAN, including NE, have beneficial anti-tumor immune effects [2, 7, 8]. We therefore sought to further study the mechanisms by which NE could contribute to favorable anti-tumor immunity.
NE has been shown to be a direct target for cancer immunotherapy. It was identified as a leukemia-associated antigen by Molldrem et al., who showed that the NE-derived human leukocyte antigen (HLA)-A2-restricted peptide, PR1 (VLQELNVTV), elicited tumor-specific cytotoxic T lymphocytes (CTLs) in patients with myeloid leukemia. Early studies showed that the presence of PR1-specific CTL (PR1-CTL) was predictive of response to treatment with allogeneic stem cell transplantation and interferon (IFN) α in patients with myeloid leukemia, and the detection of circulating PR1-CTL was correlated with improved outcomes [12]. In addition to endogenous expression by leukemia, our group has shown that non-hematopoietic cells can take up NE from the microenvironment and become susceptible to adaptive anti-tumor immune responses. Specifically, we showed that NE was cross-presented by breast cancer and melanoma cells and resulted in PR1 expression on the tumor cell surface, which enhanced the susceptibility of breast cancer and melanoma cells to lysis by PR1-targeting immunotherapies [8, 13]. We also demonstrated that NE uptake enhanced the CTL response targeting the peptide CCNE144–152, which is derived from the tumor antigen cyclin E (CCNE), a cell-cycle regulator with known prognostic value in breast cancer [7, 14, 15]. In view of these data showing NE-modulating adaptive anti-tumor immune response, we sought to elucidate the mechanisms by which this occurs.
In this report, we show that NE uptake by breast cancer cells leads to a broad increase in tumor-associated antigen (TAA) presentation. We show that enhanced antigen presentation following NE uptake by breast cancer cells is due to upregulation of HLA class I molecules on the cell surface. Furthermore, we demonstrate that the effects of NE on HLA class I extend beyond TAAs as they are also seen in the setting of viral antigens. NE-mediated upregulation of HLA class I is concentration and time-dependent, and requires NE enzymatic activity. Despite increasing HLA class I cell surface expression, NE uptake does not affect total HLA class I levels nor does it affect components of the HLA class I antigen-processing machinery, suggesting that NE uptake leads to stabilization of the HLA class I molecule on the cell surface. Taken together, our data provide a mechanism by which the innate inflammatory mediator NE enhances adaptive immune responses and identifies pathways that can be further exploited to harness the beneficial anti-tumor effects of inflammation.
Materials and methods
Patients, cells, and cell lines
Peripheral blood samples were obtained through an institutional review board-approved protocol. MDA-MB-231, MCF-7, MDA-MB-468, and SKBR3 breast cancer cell lines, the OVCAR3 ovarian cancer cell line, SW620 colon cancer cell line, H2023 non-small cell lung cancer cell line, and T2 cell line were obtained from American Type Culture Collection. MCF-HER-18 (HER18) breast cancer cell line was provided by M.C. Hung (MD Anderson Cancer Center). The MEL526 melanoma cell line was provided by L. Radvanyi (MD Anderson Cancer Center). Cell lines were validated in the institution’s sequencing and microarray facility using short tandem repeat DNA fingerprinting. The HLA-A2+ SKBR3 cell line (SKBR3-A2) was generated using lentiviral transduction, as previously described [8, 13]. Cell lines were cultured in Dulbecco’s modified Eagle’s Medium (DMEM) with 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin. T2 cells were cultured in RPMI-1640 with 10 % FBS, 100 U/mL penicillin and 100 µg/mL streptomycin. All cells were maintained in 5 % CO2 at 37 °C.
Peptide-specific CTL and cell-mediated cytotoxicity assay
Cytotoxicity assays were performed as previously described [16]. Target cells were stained with 10 µg/mL of calcein-AM (Sigma-Aldrich) for 15 min, washed, and plated in a 60-well Terasaki plate (2000 cells/10 µL RPMI 10 % human serum/well). Effector cells (E75-CTL or Epstein–Barr Virus [EBV]-CTL) were resuspended in 10 µL RPMI/10 % human serum at increasing effector to target dilutions and then added to the target cells. After 4 h, trypan blue was added as a quenching agent. Fluorescence was measured by a FLx800 Microplate Fluorescence Reader (Bio-Tek Instruments), and percent-specific cytotoxicity was determined using the following calculation: (1 − [fluorescencetarget+effector − fluorescencemedia]/[fluorescencetarget alone − fluorescencemedia]) × 100.
Target cells for cytotoxicity assays included T2 cells pulsed with E75 peptide or irrelevant control peptide, MDA-MB-231 cells, or lymphoblastoid cell lines (LCLs) maintained for 14 h ± NE (10ug/mL; Athens Research and Technology). EBV-transformed LCL was generated as previously described [17]. Briefly, peripheral blood mononuclear cells (PBMCs) were plated and treated with 1ug/mL of cyclosporine A (Sandoz), either alone for spontaneous transformation, or with supernatant derived from cultures of B cells transformed with human type 1 EBV.
E75-specific CTL (E75-CTL) was generated using a modified dendritic cell (DC) method by isolation of monocytes through adherence culturing followed by DC immunostimulation as previously described [18]. Briefly, healthy donor HLA-A2+ PBMC was allowed to adhere on 6-well plates at 37 °C in macrophage serum-free medium (Gibco). Cells remaining in suspension (lymphocytes) were removed after 4 h, pulsed with E75-peptide (40 µg/mL), and stimulated with IL-7 (10 ng/mL) and IL-2 (10 ng/mL). These cells were then maintained in RPMI 10 % FBS for 5 days. Adherent cells were matured into monocyte-derived DCs by addition of GM-CSF (100 ng/mL), IL-4 (50 ng/mL), and TNF-α (25 ng/mL). After 5 days, DCs were detached by incubation with EDTA (10 mM). DCs were pulsed with E75-peptide (40 µg/mL) for 4 h at 37 °C and then co-cultured collectively with their autologous lymphocyte population in RPMI 10 % FBS. Cells underwent re-stimulation with IL-7 (10 ng/mL) and IL-2 (25 ng/mL) for 7 days to allow for CTL proliferation. On day 12, cells were harvested and used in calcein-AM cytotoxicity assays. T2 cells, pulsed with either E75 or the irrelevant peptide PR1, were used as control target cells for E75-CTL, to confirm CTL specificity.
EBV-specific CTL (EBV-CTL) was generated as previously described [17]. Briefly, healthy donor PBMC was co-cultured with autologous irradiated LCL. After 10 days, PBMC was harvested using Ficoll gradients, subcultured, and re-stimulated with irradiated LCL. Cultures were then stimulated with IL-2 (20 U/mL; Proleukin, Cetus) 3× weekly for 3 weeks, followed by another stimulation with both IL-2 and irradiated LCL.
Cytotoxicity experiments were also performed using the anti-HLA-A2 antibody BB7.2 in order to block the HLA-A2/T cell receptor interactions and to further confirm specific killing of target cells by E75-CTL. Briefly, target cells were incubated with BB7.2 antibody for 30 min at 37 °C prior to co-culture with CTL. BB7.2 antibody was obtained from the supernatant of an established hybridoma maintained at the MD Anderson Cancer Center Monoclonal Antibody Core Facility.
Flow cytometry analysis
To evaluate NE uptake, cells were maintained in low serum media (0.5 % FBS in DMEM) ± soluble NE at various concentrations and time durations. The presence of intracellular NE was determined by permeabilizing cells using standard permeabilization buffer (BD Biosciences) and subsequent staining with Alexa Fluor 647-conjugated anti-NE antibody (clone NP-57, Santa Cruz Biotechnology) [7]. To assess cell surface HLA class I expression, cells were stained with phycoerythrin (PE)-conjugated anti-HLA-A2 (clone BB7.2; BD Biosciences) or HLA-ABC (clone G46-2.6; BD Biosciences). IFN-γ (R&D systems) treatment of cells was performed for 12 h after 24-h serum starvation at 40 ng/mL prior to treatment of cells with NE. To inhibit NE uptake by inhibition of clathrin-coated pit endocytosis, cells were treated with chlorpromazine (CPZ; Sigma-Aldrich) (30 µg/mL) for 30 min at 37 °C prior to NE supplementation. To inhibit enzymatic activity, NE was co-incubated with elafin (Sigma-Aldrich) (10 µg/mL) and phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich) (0.1 mM) for 1 h at 37 °C prior to supplementation. Protease activity was assessed using an enzymatic fluorescence-based assay (EnzChek Protease Assay; Invitrogen) according to the manufacturer’s instructions. Aqua live/dead stain (Invitrogen) was used to assess cell viability in all flow cytometry experiments, which was performed using the BD Fortessa and FACSCanto™ II flow cytometers. Data were analyzed using FlowJo software (Tree Star Inc).
Western blot analysis
Whole-cell lysates were generated from radioimmunoprecipitation assay buffer containing protease inhibitors (Santa Cruz Biotechnology). Lysates were run on 10 % SDS-PAGE gels and then subsequently transferred to polyvinylidene fluoride membranes overnight. After blocking, blots were probed for antibodies targeting HLA class I heavy chain using HC10 antibody (hybridoma provided by Dr. Bradley McIntyre, MD Anderson) or EMR8-5, (Abcam), beta-2-microglobulin (Santa Cruz Biotechnology), LMP2, transporter associated with antigen processing 1 (TAP1), calnexin and tapasin (Abcam).
Real-time quantitative PCR
Total RNA was extracted from cells using RNeasy Mini Kit (Qiagen). cDNA was synthesized using 1 µg of RNA and the iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad Laboratories). qPCRs were set up using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) following the manufacturer’s protocol. Samples were run on the CFX Connect Real-Time System (Bio-Rad Laboratories). Primer sequences used include HLA-A (forward primer 5′-GGCAGCTCAGACCACCAAGCA-3′, reverse primer 5′-CAGCGTGGTGAGTCATATGCG-3′), HLA-B (forward primer 5′-GTCCTAGCAGTTGTGGTCATC-3′, reverse primer 5′-GCTGCACGCAGCCTGAGAGTAG-3′), and HLA-C (forward primer 5′-GGTTGTCCTAGCTGTCCTTG-3′, reverse primer 5′-TCAGGCTTTACAAGTGATGAGAG-3′) and Actin, an endogenous control (forward primer 5′-TCCTGTGGCATCCACGAAACT-3′, reverse primer 5′-GAAGCATTTGCGGTGGACGAT-3′); all oligonucleotides were purchased from Sigma-Aldrich.
Statistical analysis
Comparisons between groups were made using Student’s t test and one-way ANOVA with Tukey multiple comparison test, as appropriate. p values <0.05 were considered statistically significant. Analysis was performed using Prism 6.0 software.
Results
Uptake of neutrophil elastase by breast cancer cells increases susceptibility to E75-CTL-mediated cytotoxicity
We have previously shown that NE uptake by breast cancer cells enhances their susceptibility to CCNE-CTL by increasing the cleavage of CCNE to its low molecular weight (LMW) isoforms that may be preferentially processed and presented by HLA class I molecules [7]. In addition, we have shown that after uptake by breast cancer cells, NE is cross-presented, thereby rendering breast cancer cells susceptible to killing by CTL that target the NE-derived peptide, PR1 [8]. Having shown that NE uptake increased breast cancer susceptibility to lysis by both CCNE- and PR1-CTL, we hypothesized that this phenomenon is broader with respect to NE uptake and the presentation of breast cancer TAAs. To explore this, we studied the effects of NE uptake on breast cancer susceptibility to lysis by CTL specific for the HER2-derived peptide E75. E75 is the immunodominant HLA-A2-restricted epitope of HER2, and it is the most studied of the HER2-derived peptides in both the laboratory and clinic [19]. E75-CTL was expanded from PBMC from HLA-A2+ healthy donors. Lysis was tested using cytotoxicity assays with the breast cancer cell lines MDA-MB-231, HER18, and SKBR3-A2 cultured in media ± NE. Cytotoxicity assays showed that E75-CTL was more effectively lysed cancer cells that were maintained in media that was supplemented with NE (Fig. 1). Cytotoxicity experiments were repeated utilizing the BB7.2 antibody to block HLA-A2/E75 interactions, further confirming that the observed killing was specific for the HLA-A2-restricted E75 peptide (Supplementary Figure S1). Taken together with our previous studies, these data indicate that NE has a broad effect in its ability to enhance adaptive immune responses against breast cancer antigens.
Fig. 1.
NE uptake enhances the susceptibility of breast cancer cells to lysis by E75-CTL. E75-CTL was incubated with the HLA-A2+ breast cancer cell lines MDA-MB-231, HER18, and SKBR3-A2 that were maintained in standard media ± NE (10 µg/mL) in calcein-AM cytotoxicity assays at various E (effector): T (target) ratios. NE uptake by all three cell lines resulted in their increased lysis by E75-CTL. Assays were performed in triplicate; results are representative of 5 separate experiments. *p < 0.05, **p < 0.01, ***p < 0.001 comparing NE treated to untreated
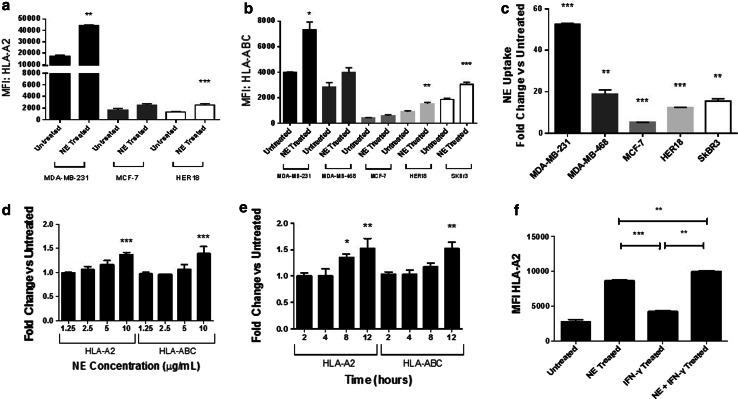
Uptake of neutrophil elastase by breast cancer cells leads to increased cell surface HLA class I expression
Having shown that NE uptake enhances the killing of breast cancer cells by CTL specific for multiple different antigens, we next sought to investigate the mechanism by which this occurs. In view of our data showing an increase in PR1 and CCNE144–152 presentation by HLA [7, 8], we studied the effects of NE on HLA class I presentation. Various breast cancer cell lines, including the HLA-A2 positive MDA-MB-231, MCF-7, and HER18 cell lines, and the HLA-A2 negative MDA-MB-468 and SKBR3 cell lines, were maintained in standard media ± NE for 12 h. After confirming NE uptake, we examined the cells for HLA-A2 surface expression as well as all HLA class I molecules (HLA-ABC) using flow cytometry. As shown in Fig. 2a, b, NE uptake by MDA-MB-231 and HER18 cells led to a significant increase in the cell surface expression of HLA-A2 and HLA-ABC. Similarly, NE uptake by SKBR3 cells (HLA-A2 negative) led to a significant increase HLA-ABC expression. The extent of NE uptake is shown in Fig. 2c.
Fig. 2.
NE uptake leads to increased surface expression of HLA class I in breast cancer. MDA-MB-231, MDA-MB-468, MCF-7, HER18, and SKBR3 breast cancer cells were maintained in standard media ± NE (10 µg/mL). a–c NE uptake corresponded with an increase in HLA-ABC and HLA-A2 on the cell surface. d, e NE uptake in MDA-MB-231 cells led to concentration-dependent and time-dependent increases in surface expression of HLA-A2 and HLA-ABC. f IFN-γ treatment of MDA-MB-231 cells led to an increase in HLA-A2 compared to untreated cells. The addition of NE further increased HLA-A2 surface expression compared to IFN-γ treatment alone. Experiments were performed in triplicate; results are representative of 3 separate experiments. *p < 0.05, **p < 0.01, ***p < 0.001 comparing NE treated to untreated, unless designated; MFI, median fluorescence intensity
To further investigate whether the degree of NE uptake correlates with the increase in HLA class I expression, we repeated experiments using non-breast cancer cell lines, including MEL526 melanoma, H2023 non-small cell lung cancer, OVCAR3 ovarian cancer, and SW620 colon cancer cells lines. The impact of NE uptake on cell surface HLA class I expression was confirmed in the MEL526 and H2023 tumor cell lines (Supplementary Figures S2a and S2b) but not in the OVCAR3 and SW620 tumor cell lines (Supplementary Figure S2c and S2d). Taken together, these data confirm what we, and others, have shown specifically that NE can be taken up by multiple solid tumor types [5, 8]. In addition, it shows that in some tumor types, NE uptake leads to increased cell surface HLA class I expression but that the degree of uptake does not correlate with the extent of HLA class I expression. This observation points to distinct intracellular mechanisms that are regulated by NE, depending on the cell type.
Because the MDA-MB-231 breast cancer cell line showed the highest level of NE uptake with a considerable increase in HLA class I surface expression, this cell line was used to further investigate the mechanism by which NE uptake regulates antigen presentation. We sought to determine whether the effect of NE on HLA class I was concentration- and time-dependent. First, cells were maintained in standard media supplemented with varying concentrations of NE, ranging from 1.25 to 10 µg/mL, for 12 h. Cell surface staining showed a concentration-dependent increase in both HLA-A2 and HLA-ABC after NE uptake (Fig. 2d). Next, MDA-MB-231 cells were maintained in media supplemented with NE (10 µg/mL) for various time points ranging from 2 to 12 h. We found an increase in HLA-A2 and HLA-ABC cell surface expression over time, corresponding to NE uptake (Fig. 2e). Also, NE uptake peaked at 4 h and remained significantly elevated up to the last time point measured (12 h; Supplementary Figure S3a). Together, these data demonstrate that the effect of NE uptake on HLA class I surface expression is both concentration- and time-dependent.
Since NE is one component of a complex inflammatory milieu, and having shown the isolated effects of NE on HLA class I expression, we next examined the effects of NE and IFN-γ on cell surface HLA class I. IFN-γ is an important inflammatory response mediator that has been shown to increase HLA class I expression in solid tumors [20]. MDA-MB-231 cells were treated with IFN-γ and then maintained in standard media ± NE. Consistent with previous reports, IFN-γ treatment led to increased HLA-A2 cell surface expression. The effect of NE uptake on HLA-A2 cell surface expression was greater than that of IFN-γ (p < 0.001), and the combination of IFN-γ and NE led to a greater increase in HLA-A2 cell surface expression than either IFN-γ or NE alone (Fig. 2f). Consistent with our experiments showing an association between NE uptake and the regulation of HLA expression, IFN-γ treatment enhanced NE uptake by MDA-MB-231 cells (Supplementary Figure S3b), which could explain the synergistic effects on HLA class I expression in the cells treated with both NE and IFN- γ. Experiments were repeated using MDA-MB-468 and MCF7 cells. For MDA-MB-468 cells, which take up NE, the results were consistent with those seen with MDA-MB-231 cells. Specifically, the effect of NE uptake on HLA-ABC cell surface expression was greater than that of IFN-γ, and the combination of IFN-γ and NE led to a greater increase in HLA-ABC cell surface expression than either IFN-γ or NE alone (Supplementary Figure S4a). Consistent with our experiments that suggest an association between NE uptake and HLA increase in some breast cancer cell lines, there was no significant increase in HLA-A2 expression in MCF-7 cells that were cultured in NE-supplemented media, but there was a significant increase in HLA-A2 expression after the addition of IFN-γ (Supplementary Figure S4b).
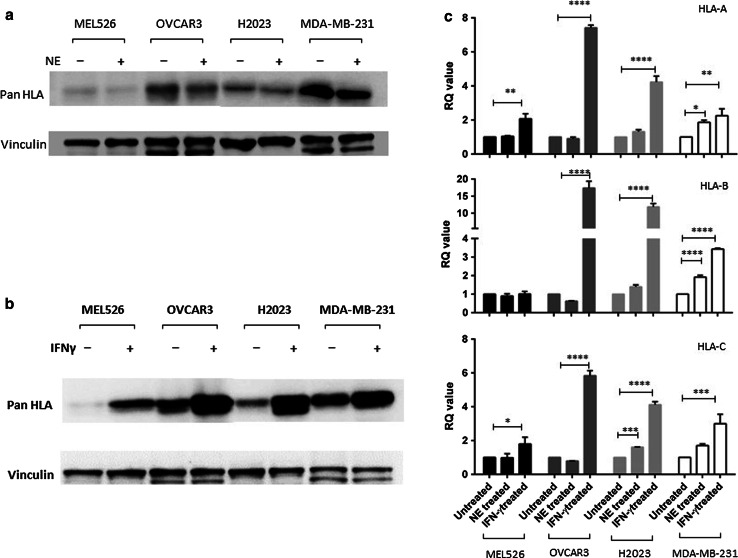
Increased cell surface HLA class I expression is not due to an increase in total HLA class I protein expression
In order to determine whether changes in cell surface HLA class I expression were due to an increase in total HLA class I protein expression, Western blot analysis was performed on lysates from MDA-MB-231, MEL526, OVCAR3 and H2023 cells maintained in standard media or media supplemented with either NE or IFN-γ. As shown in Fig. 3a, there was no change in total HLA class I protein expression in cells maintained in media supplemented with NE. In contrast, there was an increase in total HLA class I protein expression in cells maintained in media supplemented with IFN-γ (Fig. 3b). Concomitantly, there was an increase in the transcripts as demonstrated by qPCR in cells maintained in IFNγ-supplemented media but not in those maintained in NE-supplemented media (Fig. 3c). These data suggest that the effect of NE uptake on HLA class I expression is post-transcriptionally regulated.
Fig. 3.
Increased cell surface HLA class I expression is not due to an increase in total HLA class I protein expression. MEL526, OVCAR3, H2023 and MDA-MB-231 cells were maintained in media supplemented with either NE or IFN-γ. a Western blot analysis showed no change in HLA class expression in any of the cell lines after NE uptake. b In contrast, Western blot analysis showed increased HLA class I expression in all cells lines maintained in media supplemented with IFN-γ. c RT-qPCR evaluating HLA-A, B, or C mRNA was carried out using RNA extracted from MEL526, OVCAR3, H2023 and MDA-MB-231 cells maintained in media supplemented with either NE or IFN-γ. For cells maintained in NE-supplemented media, there was no change, suggesting that HLA class I expression is post-transcriptionally regulated. In contrast, for cells maintained in IFN-γ-supplemented media, there was an increase in HLA-A, B and C transcripts suggesting transcriptional regulation. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
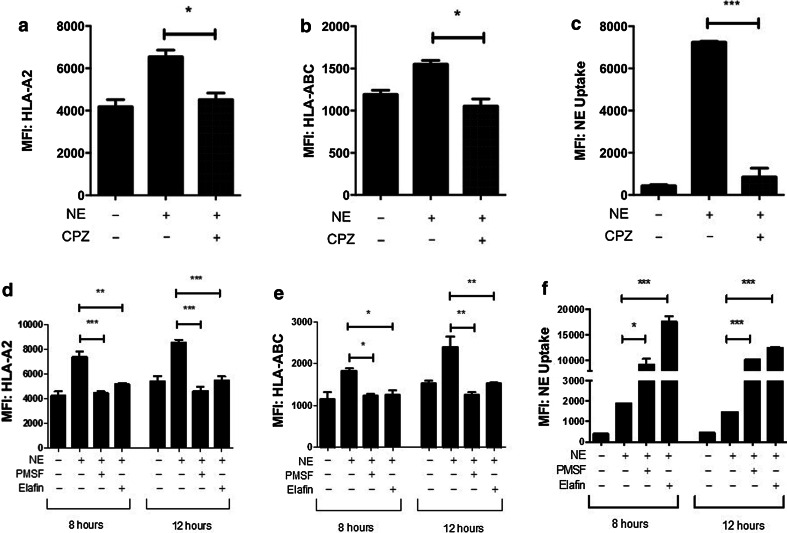
Uptake of enzymatically active NE is required for HLA class I regulation
To further understand the mechanism of NE-mediated upregulation of HLA class I surface expression, we studied whether NE uptake was required for the demonstrated increase. NE has been shown to have downstream effects on tumor cell proliferation and migration via extracellular mechanisms [21]. Alternatively, published reports and our data have shown uptake-dependent effects of NE on tumor cell proliferation and antigen presentation [5, 8, 22]. Thus, it is important to delineate whether NE uptake was required for its regulation of HLA class I. To investigate this, we used CPZ, which inhibits clathrin-coated pit-mediated endocytosis [23, 24], which was shown to play a role in NE uptake by solid tumors [22]. MDA-MB-231 cells were treated with CPZ prior to culture in NE-supplemented media for 8 h. CPZ treatment resulted in inhibition of NE uptake with a subsequent significant attenuation in the NE-mediated increase in HLA-A2 and HLA-ABC cell surface expression (Fig. 4a–c), demonstrating that HLA class I upregulation by NE requires its uptake by the breast cancer cell.
Fig. 4.
Uptake of enzymatically active NE is required for the regulation of HLA class I by NE. a–c CPZ treatment abrogated the effects of NE on HLA-A2 and HLA-ABC surface expression by inhibiting NE uptake. d, e Inhibition of NE enzymatic activity by co-culture of NE with either elafin or PMSF abrogated the increase in HLA-A2 and HLA-ABC expression in MDA-MB-231 cells. f Enzymatic inhibition of NE following the incubation of MDA-MB-231 cells with PMSF or elafin resulted in increased NE uptake compared with enzymatically active NE. Experiments were performed in triplicate; results are representative of 3 separate experiments. *p < 0.05; **p < 0.01; ***p < 0.001; MFI median fluorescence intensity, PMSF phenylmethanesulfonyl fluoride, CPZ chlorpromazine
We next sought to determine whether the protease activity of NE was required to produce the effect on HLA expression. We have previously shown that NE co-incubation with elafin, a natural reversible inhibitor of NE [25], inhibits the enzymatic activity of NE without decreasing uptake by MDA-MB-231 cells [7]. Incubation of NE with either elafin or PMSF, a serine protease inhibitor known to covalently bind NE [26], abrogated the effect of NE on HLA-A2 and HLA-ABC surface expression in MDA-MB-231 cells (Fig. 4d, e). This was observed despite the further increase in NE uptake following enzyme inhibition (Fig. 4f). Inhibition of enzymatic activity was confirmed using a fluorescence-based enzymatic assay (Supplementary Figure S5).
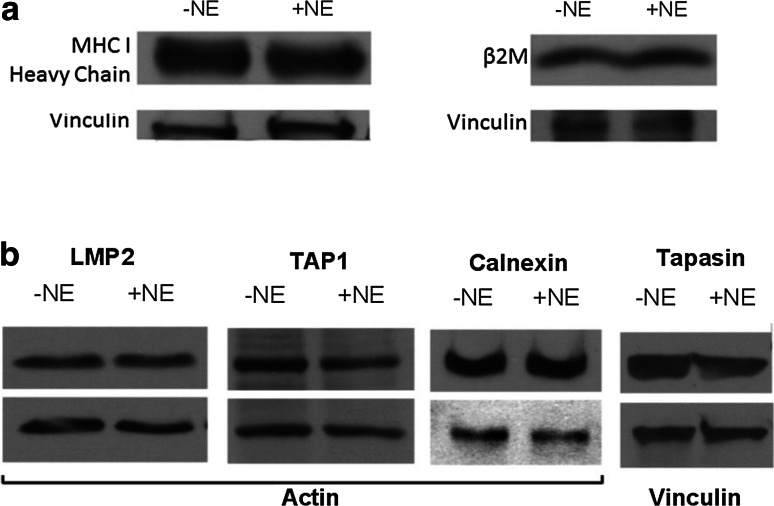
NE uptake by breast cancer cells does not affect the antigen-processing machinery
Having shown that NE uptake and enzymatic activity are required for increasing HLA class I cell surface expression, we next investigated how NE uptake affects the antigen-processing machinery (APM). The HLA class I APM requires sequential steps including proteasomal cleavage of the antigen to generate peptides that subsequently enter the endoplasmic reticulum (ER) where they are folded with the aid of ER chaperones and finally coupled with HLA class I molecules for transport to the cell surface [27]. The effects of NE uptake on specific components of the APM were therefore investigated as previously described [28–30]. Whole-cell lysates were harvested from MDA-MB-231 cells after 12 h of culture in NE-supplemented media and then analyzed by western blot for HLA class I heavy chain, beta-2-microglobulin (β2M), the proteasomal subunit LMP2, the ER transporter TAP1 and ER chaperones calnexin and tapasin. Consistent with our Western blot data showing no effects of NE uptake on HLA class I total protein using the HLA class I antibody HC10, which binds to the alpha 3 domain of the HLA complex, we found that NE uptake also did not affect the β2M component of the HLA molecule or the HLA heavy chain using another anti-HLA class I antibody (EMR8-5) (Fig. 5a). Furthermore, NE uptake did not lead to changes in expression in any other components of the APM throughout all the time points examined, ranging from 1 to 48 h (12-h time point is shown in Fig. 5b). Taken together, these data suggest that the increased cell surface expression of HLA class I seen in tumor cells after NE uptake is likely due to cell surface stabilization of HLA class I molecules, rather than an increase in the total levels of HLA class I molecules or components of the APM.
Fig. 5.
NE uptake does not affect the HLA class I antigen-processing machinery. a Western blot analysis showed no change in expression of HLA class I heavy chain or beta-2-microglobulin (β2M) in MDA-MB-231 cells after NE uptake. b NE did not affect APM components, including LMP2 (23 kDa), TAP1 (81 kDa), calnexin (75 kDa), and tapasin (50 kDa). Actin or vinculin was used as loading controls. Results are representative of 3 separate experiments. APM, antigen-processing machinery
Discussion
In this report, we identify a broad function of NE in its ability to enhance killing of breast cancer cells by tumor antigen-specific CTL. Mechanistically, this involves increasing cell surface expression of HLA class I molecules, a process that requires NE uptake and enzymatic activity. Furthermore, we show that the regulation of HLA class I by NE is not due to an increase in total HLA class I or components of the APM. Because NE uptake does not affect total HLA class I expression or expression of components of the APM, these data suggest that NE stabilizes the HLA class I molecule on the cell surface. Finally, we demonstrate that the effects of NE on HLA class I and antigen presentation extend beyond tumor antigens to viral antigens. Together, our data demonstrate a novel role of NE impacting adaptive immune responses by broadly increasing antigen presentation.
Antigen processing and presentation are critical in generating adaptive anti-tumor immune responses. In fact, the downregulation of HLA class I and components of the APM are mechanisms by which tumors evade the immune system [31–34]. It has previously been described that there may be irreversible and reversible mechanisms responsible for HLA class I downregulation [35]. If the downregulation of HLA class I is irreversible (i.e., due to a structural defect), then the escape mechanism will not be overcome and the tumor will remain “invisible” to the immune system. In this scenario, we predict that NE would not have an effect on the HLA class I. Conversely, if the mechanism of HLA class I downregulation is reversible, then it is possible that HLA class I expression can be upregulated by inflammatory products such as cytokines and NE, so that antigen-specific CTL responses will increase leading to tumor regression. Inflammation, specifically products of the innate immune response, is critical to shaping the adaptive immune response targeting cancer cells in part by its regulation of HLA and the APM in tumor cells. For example, type I and II IFNs have been shown to play major roles in shaping anti-tumor immunity by increasing the expression of HLA class I and components of the APM [17, 36–38]. Here, we report the important observation that NE uptake results in increased HLA class I expression. We focused on NE because our previous data have linked NE to enhanced anti-tumor immune responses to CCNE- and PR-1 specific CTL, and because NE is produced by neutrophils and macrophages, which are both major components of the tumor inflammatory microenvironment [7, 8]. In this report, we have defined a novel role for NE as a regulator of adaptive immunity by its effects on HLA class I antigen presentation.
Our data suggest that the mechanism of NE enhancement of adaptive immunity is primarily driven by its effects on cell surface antigen presentation by HLA class I. CTL immunogenicity is strongly influenced by the stability of the peptide–HLA class I complex, which is dependent on peptide availability [39–41]. Since we showed NE-mediated time- and concentration-dependent increases in HLA class I on the cell surface, with no change in total HLA class I expression or the APM (Fig. 4), we conclude that the primary mechanism by which NE exerts its effects on HLA class I is by stabilizing the HLA molecules on the cell surface. The data presented in this report along with our previous work together suggest that NE enhances the generation of HLA class I-restricted peptides by directly serving as a source antigen for immunogenic peptides (i.e., PR1) [8] or by cleaving existing antigens into molecular forms that are more favorably presented by the HLA class I (i.e., LMW-CCNE) [7]. This is strongly supported by the data in this report, showing that inhibition of endosomal uptake by CPZ or inhibition of the enzymatic activity of NE (Fig. 3) abrogates the effect of NE on HLA class I. Whether NE enhances the presentation of naturally presented epitopes or generates neo-epitopes is the subject of ongoing studies by our group.
Although a number of reports have linked inflammation with positive anti-tumor effects [2, 7, 8, 42], other reports have correlated inflammation with poor clinical outcomes [4, 6]. For example, while Eruslanov et al. [2] showed that TAN produce proinflammatory cytokines and costimulatory molecules that enhanced anti-tumoral T cell responses in lung cancer, Houghton et al. [5, 22] showed that the uptake of NE by solid tumors, including lung cancer, leads to degradation of insulin receptor substrate-1 in tumor cells, which activates the phosphoinositide 3-kinase pathway ultimately promoting tumor cell proliferation. Although our work does not provide an explanation for the dichotomous reports where inflammation is beneficial in some tumors and deleterious in others, our report, along with previous studies, highlights the importance of individual components of the tumor inflammatory microenvironment that could be targeted to enhance (or attenuate) the anti-tumoral (or pro-tumoral) immune effects.
Multiple mechanisms for immune escape have been described; some are intrinsic to the activated tumor-infiltrating T cells, while others are extrinsic to the T cells and include cellular and non-cellular elements within the tumor microenvironment (reviewed by Mittendorf and Sharma [43]). Among the intrinsic mechanisms are downregulation of T cell costimulatory molecules, increased expression of T cell inhibitory molecules, loss of tumor-specific antigens as well as downregulation of components of the APM and HLA class I [31–34, 44, 45]. As discussed above, there are mechanisms of HLA class I downregulation that may be reversible [35]. Consistent with this, our findings suggest that there may be a potential to exploit the favorable effects of NE on HLA class I and antigen presentation to strategically enhance existing immunotherapies and to develop novel approaches to targeting tumor antigens, thereby overcoming immune escape. For example, the efficacy of peptide vaccines and adoptive cellular therapies, which depends on the presentation of the antigen by the tumor cell for recognition by the antigen-specific CTL, may be augmented by enhancing uptake of NE from the tumor microenvironment, while controlling for factors that negatively contribute to tumor cell proliferation. This approach would have implications that could improve the efficacy of peptide vaccines and adoptive cellular therapies. Our findings could also have implications for determining the tumor types that may be susceptible to targeted immunotherapy approaches based on their degree of inflammation and NE expression. Together, our data have shown a novel mechanism through which inflammation could have anti-tumor immune effects that can be harnessed to improve the efficacy of cancer immunotherapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by Grant R00CA133244 from the National Cancer Institute (NCI) (to Elizabeth A. Mittendorf), Leukemia SPORE CA100632 (to Jeffrey J. Molldrem), NCI P01 CA148600-01 (to Jeffrey J. Molldrem), Leukemia and Lymphoma Society Research Grant #6030-12 (to Jeffrey J. Molldrem), the T32 CA009599 training grant (to Akhil Chawla and Victor Gall) and the T32 CA009598 training grant (to Celine Kerros and Haley L. Peters) from the NCI. The Flow Cytometry and Cellular Imaging core were supported by Cancer Center Support Grant NCI #CA16672. STR DNA fingerprinting was done by the Cancer Center Support Grant funded Characterized Cell Line core, NCI #CA16672. Elizabeth A. Mittendorf is an R. Lee Clark Fellow of the University of Texas MD Anderson Cancer Center supported by the Jeanne F. Shelby Scholarship Fund.
Abbreviations
- APM
Antigen-processing machinery
- CCNE
Cyclin E
- CPZ
Chlorpromazine
- CTLs
Cytotoxic T lymphocytes
- DC
Dendritic cell
- DMEM
Dulbecco’s modified Eagle’s Medium
- EBV
Epstein–Barr virus
- ER
Endoplasmic reticulum
- FBS
Fetal bovine serum
- HLA
Human leukocyte antigen
- IFN
Interferon
- LCLs
Lymphoblastoid cell lines
- LMW
Low molecular weight
- NE
Neutrophil elastase
- PBMCs
Peripheral blood mononuclear cells
- PE
Phycoerythrin
- PMSF
Phenylmethanesulfonyl fluoride
- TAA
Tumor-associated antigen
- TAMs
Tumor-associated macrophages
- TANs
Tumor-associated neutrophils
- TNF
Tumor necrosis factor
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Footnotes
Gheath Alatrash and Elizabeth A. Mittendorf have contributed equally to this work.
Contributor Information
Gheath Alatrash, Email: galatras@mdanderson.org.
Elizabeth A. Mittendorf, Email: eamitten@mdanderson.org
References
- 1.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 4.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 5.Houghton AM, Rzymkiewicz DM, Ji H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foekens JA, Ries C, Look MP, Gippner-Steppert C, Klijn JG, Jochum M. The prognostic value of polymorphonuclear leukocyte elastase in patients with primary breast cancer. Cancer Res. 2003;63:337–341. [PubMed] [Google Scholar]
- 7.Mittendorf EA, Alatrash G, Qiao N, et al. Breast cancer cell uptake of the inflammatory mediator neutrophil elastase triggers an anticancer adaptive immune response. Cancer Res. 2012;72:3153–3162. doi: 10.1158/0008-5472.CAN-11-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alatrash G, Mittendorf EA, Sergeeva A, et al. Broad cross-presentation of the hematopoietically derived PR1 antigen on solid tumors leads to susceptibility to PR1-targeted immunotherapy. J Immunol. 2012;189:5476–5484. doi: 10.4049/jimmunol.1201221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Pelt LJ, Huisman MV, Weening RS, von dem Borne AE, Roos D, van Oers RH. A single dose of granulocyte-macrophage colony-stimulating factor induces systemic interleukin-8 release and neutrophil activation in healthy volunteers. Blood. 1996;87:5305–5313. [PubMed] [Google Scholar]
- 10.van der Poll T, Jansen PM, Van Zee KJ, et al. Tumor necrosis factor-alpha induces activation of coagulation and fibrinolysis in baboons through an exclusive effect on the p55 receptor. Blood. 1996;88:922–927. [PubMed] [Google Scholar]
- 11.Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5:182–190. doi: 10.1016/S1470-2045(04)01414-7. [DOI] [PubMed] [Google Scholar]
- 12.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 13.Alatrash G, Ono Y, Sergeeva A, et al. The role of antigen cross-presentation from leukemia blasts on immunity to the leukemia-associated antigen PR1. J Immunother. 2012;35:309–320. doi: 10.1097/CJI.0b013e31824b3b14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 15.Desmedt C, Ouriaghli FE, Durbecq V, et al. Impact of cyclins E, neutrophil elastase and proteinase 3 expression levels on clinical outcome in primary breast cancer patients. Int J Cancer. 2006;119:2539–2545. doi: 10.1002/ijc.22149. [DOI] [PubMed] [Google Scholar]
- 16.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, Fukushima P, Barrett AJ. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. [PubMed] [Google Scholar]
- 17.Smith CA, Ng CY, Heslop HE, et al. Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. J Hematother. 1995;4:73–79. doi: 10.1089/scd.1.1995.4.73. [DOI] [PubMed] [Google Scholar]
- 18.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Evaluation of the CD107 cytotoxicity assay for the detection of cytolytic CD8+ cells recognizing HER2/neu vaccine peptides. Breast Cancer Res Treat. 2005;92:85–93. doi: 10.1007/s10549-005-0988-1. [DOI] [PubMed] [Google Scholar]
- 19.Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57:1511–1521. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Propper DJ, Chao D, Braybrooke JP, et al. Low-dose IFN-gamma induces tumor MHC expression in metastatic malignant melanoma. Clin Cancer Res. 2003;9:84–92. [PubMed] [Google Scholar]
- 21.Wada Y, Yoshida K, Tsutani Y, et al. Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol Rep. 2007;17:161–167. [PubMed] [Google Scholar]
- 22.Gregory AD, Hale P, Perlmutter DH, Houghton AM. Clathrin pit-mediated endocytosis of neutrophil elastase and cathepsin G by cancer cells. J Biol Chem. 2012;287:35341–35350. doi: 10.1074/jbc.M112.385617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su H, Liu B, Frohlich O, Ma H, Sands JM, Chen G. Small GTPase Rab14 down-regulates UT-A1 urea transport activity through enhanced clathrin-dependent endocytosis. FASEB J. 2013;27:4100–4107. doi: 10.1096/fj.13-229294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying QL, Simon SR. Kinetics of the inhibition of human leukocyte elastase by elafin, a 6-kilodalton elastase-specific inhibitor from human skin. Biochemistry. 1993;32:1866–1874. doi: 10.1021/bi00058a021. [DOI] [PubMed] [Google Scholar]
- 26.Mark DE, Lazzari KG, Simons ER. Are serine proteases involved in immune complex activation of neutrophils? J Leukoc Biol. 1988;44:441–447. doi: 10.1002/jlb.44.5.441. [DOI] [PubMed] [Google Scholar]
- 27.Heemels MT, Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 28.Leone P, Shin EC, Perosa F, Vacca A, Dammacco F, Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst. 2013;105:1172–1187. doi: 10.1093/jnci/djt184. [DOI] [PubMed] [Google Scholar]
- 29.Giorda E, Sibilio L, Martayan A, et al. The antigen processing machinery of class I human leukocyte antigens: linked patterns of gene expression in neoplastic cells. Cancer Res. 2003;63:4119–4127. [PubMed] [Google Scholar]
- 30.Krishnakumar S, Abhyankar D, Sundaram AL, Pushparaj V, Shanmugam MP, Biswas J. Major histocompatibility antigens and antigen-processing molecules in uveal melanoma. Clin Cancer Res. 2003;9:4159–4164. [PubMed] [Google Scholar]
- 31.Campoli M, Chang CC, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;20(Suppl 4):A40–A45. doi: 10.1016/S0264-410X(02)00386-9. [DOI] [PubMed] [Google Scholar]
- 32.Delp K, Momburg F, Hilmes C, Huber C, Seliger B. Functional deficiencies of components of the MHC class I antigen pathway in human tumors of epithelial origin. Bone Marrow Transplant. 2000;25(Suppl 2):S88–S95. doi: 10.1038/sj.bmt.1702363. [DOI] [PubMed] [Google Scholar]
- 33.Ogino T, Shigyo H, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, Ferrone S. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 34.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–464. doi: 10.1016/S0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 35.Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–256. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]
- 36.Seliger B, Hammers S, Hohne A, Zeidler R, Knuth A, Gerharz CD, Huber C. IFN-gamma-mediated coordinated transcriptional regulation of the human TAP-1 and LMP-2 genes in human renal cell carcinoma. Clin Cancer Res. 1997;3:573–578. [PubMed] [Google Scholar]
- 37.Yang I, Kremen TJ, Giovannone AJ, Paik E, Odesa SK, Prins RM, Liau LM. Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex-bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J Neurosurg. 2004;100:310–319. doi: 10.3171/jns.2004.100.2.0310. [DOI] [PubMed] [Google Scholar]
- 38.Dunn IS, Haggerty TJ, Kono M, Durda PJ, Butera D, Macdonald DB, Benson EM, Rose LB, Kurnick JT. Enhancement of human melanoma antigen expression by IFN-beta. J Immunol. 2007;179:2134–2142. doi: 10.4049/jimmunol.179.4.2134. [DOI] [PubMed] [Google Scholar]
- 39.van der Burg SH, Visseren MJ, Brandt RM, Kast WM, Melief CJ. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J Immunol. 1996;156:3308–3314. [PubMed] [Google Scholar]
- 40.Busch DH, Pamer EG. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J Immunol. 1998;160:4441–4448. [PubMed] [Google Scholar]
- 41.Harndahl M, Rasmussen M, Roder G, Dalgaard Pedersen I, Sorensen M, Nielsen M, Buus S. Peptide-MHC class I stability is a better predictor than peptide affinity of CTL immunogenicity. Eur J Immunol. 2012;42:1405–1416. doi: 10.1002/eji.201141774. [DOI] [PubMed] [Google Scholar]
- 42.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 43.Mittendorf EA, Sharma P. Mechanisms of T-cell inhibition: implications for cancer immunotherapy. Expert Rev Vaccines. 2010;9:89–105. doi: 10.1586/erv.09.144. [DOI] [PubMed] [Google Scholar]
- 44.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chawla A, Philips AV, Qiao N, Sukhumalchandra P, Kerros C, Molldrem J, Alatrash G, Mittendorf EA. Neutrophil elastase uptake by breast cancer increases anti-tumor adaptive immune response by upregulation of HLA class I molecules. [Abstract P21] Ann Surg Oncol. 2014;21:S51. doi: 10.1245/s10434-013-3229-6. [DOI] [Google Scholar]
- 47.Chawla A, Philips AV, Qiao N, Sukhumalchandra P, Kerros C, Alatrash G, Molldrem JJ, Mittendorf EA (2014) Neutrophil elastase enhances adaptive immunity via increase of peptide presentation and downregulation of T cell inhibitory molecules [Abstract 4843/11] In: Proceedings of the 105th annual meeting of the American Association for Cancer Research, 2014 Apr 5–9, San Diego, CA. Philadelphia (PA): AACR
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.