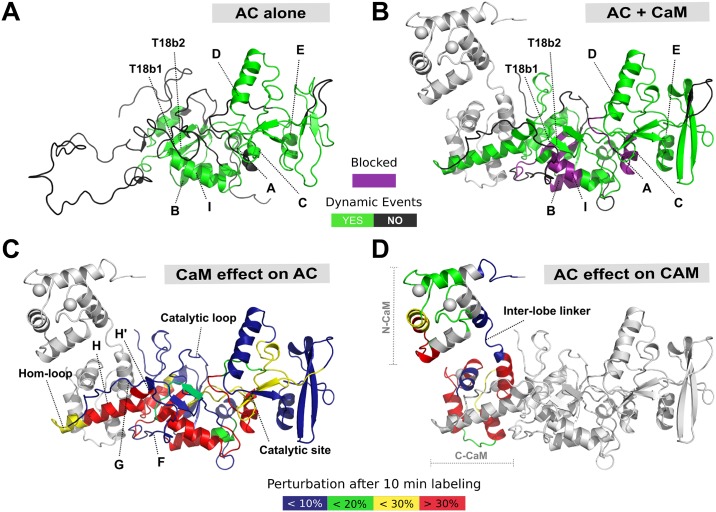
Fig 6. The structural interplay of AC and CaM complex formation.
Specific regions within the AC T18 domain serve as a MoRF for CaM recognition, binding, and activation of AC itself. (A) In the absence of CaM, the F-, G-, H-, and H′-helices and Hom-loop are found as an extended disordered coil, acting as a bait for CaM capture. (B) An interplay between protein structural disorder and order is requisite for activation by CaM of AC catalytic function. Upon CaM binding, the H- and H′-helices undergo extensive structure formation, resulting in a conformation that is appropriate for catalytic activation. Helices F and G and the Hom-loop remain unstructured throughout. Some regions become “blocked” and resistant to deuteration (highlighted in purple). (C) The effect of CaM binding on AC. (D) The effect of AC on CaM. CaM binding to AC results in widespread perturbations, primarily within the T18 region of the protein, while AC primarily binds to C-CaM, with only a transient interaction in N-CaM. In addition to those effects occurring in the H/H′ region, long-range allosteric remodeling is observed at the site of catalysis, which becomes more stable and rigid. Meanwhile, the catalytic loop does not undergo any dramatic structural rearrangement, remaining unstructured and exposed regardless of CaM availability. This is suited to a maximal turnover of ATP substrate and thus maximal toxicity in the form of cAMP production. The data used to generate the figure can be found in S1, S3 and S4 Data. AC, adenylate cyclase catalytic domain; C-CaM, C-terminal domain of CaM; CaM, calmodulin; MoRF, molecular recognition feature; N-CaM, N-terminal domain of CaM; T18, C-terminal trypsin-cleavage fragment of CyaA (amino acids 225–364); T18b1, first beta-sheet of the T18 fragment; T18b2, second beta-sheet of the T18 fragment.