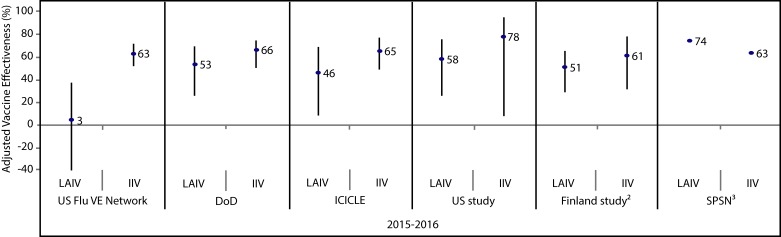
Figure 2. Adjusted vaccine effectiveness estimates against any influenza by study and vaccine type for the 2015–2016 influenza season in children and adolescents 2–17 years of age1.
Abbreviations: DoD, United States Department of Defense; ICICLE, Influenza Clinical Investigation for Children study; IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine; SPSN, Canadian Sentinel Practitioner Surveillance Network; UK Study, United Kingdom Study; US FLU VE Network, United States Influenza Vaccine Effectiveness Network; %, percentage 1 For each study in the forest plot, the black circle represents the vaccine effectiveness point estimate and the vertical bar represents the corresponding 95% confidence interval. The 95% confidence interval lower limits are truncated at -40% 2 The Finland national cohort study reported vaccine effectiveness in children two years of age 3 The Canadian SPSN reported wide and overlapping 95% confidence intervals (exact values not publicly available at time of writing)