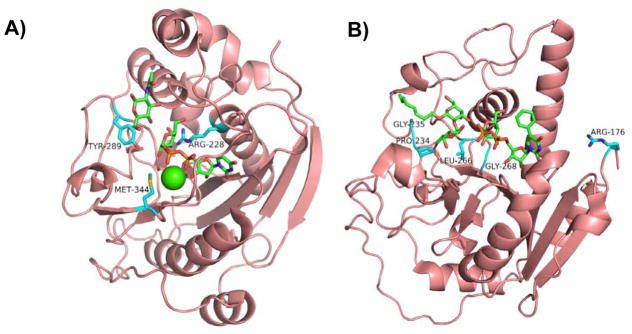
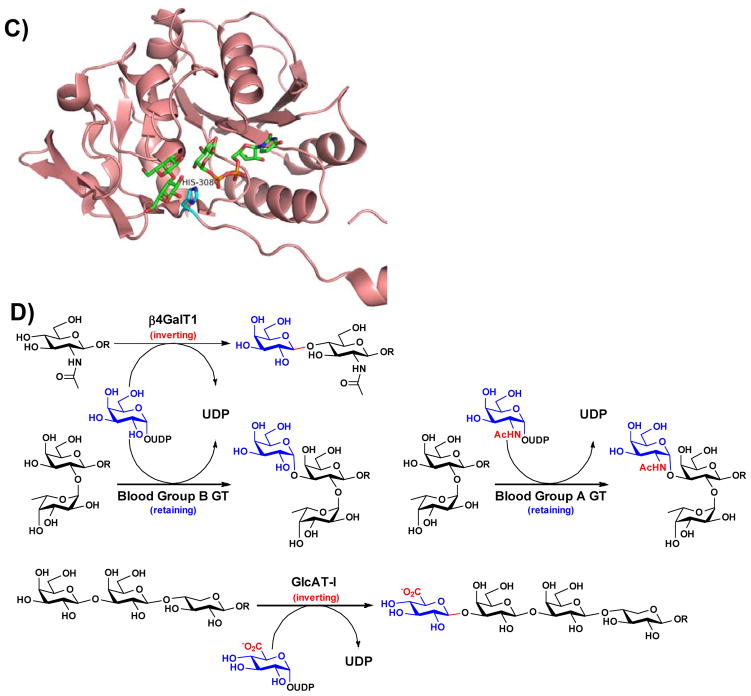
Figure 2.
Structures of representative GT-A-fold enzymes (A–C) highlighting the residues known to influence their substrate specificities as well as reactions (D) catalyzed by these enzymes. (A) Bovine β4GalT1 (PDB IDs: 1NQI and 2FYC) with GlcNAc, UDP-Gal (1), and Ca2+; (B) Human blood group antigen A GT (PDB IDs: 1R81, 3V0L, and 3V0Q) with UDP-Gal (1) analog and H-antigen acceptor; (C) Human β3GlcAT-I (PDB IDs: 1FGG and 1KWS) with Galβ1–3Gal and UDP-GlcA (9). Carbon atoms in the donor and acceptor substrates are shown in green; carbon atoms in amino acid residues are shown in cyan; oxygen atoms are shown in red; nitrogen atoms are shown in blue; and sulfur atom is shown in gold. Images contain elements from multiple PDB structures aligned in PyMOL. (D) β4GalT1 is an inverting GT which catalyzes the transfer of Gal from UDP-Gal (in an α-linkage) with an inversion of stereochemistry at the anomeric carbon of Gal to form β-linked galactosides. Human blood group B GT catalyzes the transfer of Gal from UDP-Gal with a retention of anomeric stereochemistry to form α-linked galactosides. Human blood group A GT is a retaining GT catalyzing the transfer of GalNAc from UDP-GalNAc to blood H antigen acceptor. β3GlcAT-I is an inverting GT catalyzing the transfer of GlcA from UDP-GlcA to Galβ1–3Galβ1–4-Xyl for the formation of the tetrasaccharide core structure of glycosaminoglycans in proteoglycans.