Abstract
Spinal cord injury (SCI) triggers chronic intraspinal inflammation consisting of activated resident and infiltrating immune cells (especially microglia/macrophages). The environmental factors contributing to this protracted inflammation are not well understood, however, myelin lipid debris is a hallmark of SCI. Myelin is also a potent macrophage stimuli and target of complement-mediated clearance and inflammation. The downstream effects of these neuro-immune interactions have the potential to contribute to ongoing pathology or facilitate repair. This depends in large part on whether myelin drives pathological or reparative macrophage activation states, commonly referred to as M1 (pro-inflammatory) or M2 (alternatively) macrophages respectively. Here we review the processes by which myelin debris may be cleared through macrophage surface receptors and the complement system, how this differentially influences macrophage and microglial activation states, and how the cellular functions of these myelin macrophages and complement proteins contribute to chronic inflammation and secondary injury after SCI.
Keywords: Marco, TREM2, C1q, CR3, scavenger receptor AI/II, CD36
Introduction
Spinal cord injury (SCI) triggers a complex cross-talk between resident cells of the central nervous system (CNS) and infiltrating immune cells. These neuro-immune interactions can mediate recovery but also inhibit regeneration. Activated macrophages, consisting of resident microglia and recruited monocytes, contribute to this dichotomous response. Indeed, macrophages facilitate repair by increasing axon growth, stem cell differentiation, and revascularization. However, macrophages can also contribute to pathology through reactive oxygen species (ROS), neurotoxin, and pro-inflammatory cytokines release, as well as, by causing axon retraction and dieback. The extent to which macrophages are polarized toward reparative (also called M2 or alternative) or pathological (also called M1 or pro-inflammatory) phenotypes depends in large part on the stimuli present in the injured spinal cord.
While numerous studies examine how macrophage activation states affect recovery after SCI (for a review see (Gensel and Zhang, 2015)), less is understood about how the lesion environment contributes to macrophage polarization. It is well-established that myelin debris generated after SCI inhibits axonal regeneration and remyelination (McKerracher et al., 1994), however myelin can also act as an inflammatory stimulus (Kroner et al., 2014; Wang et al., 2014; Williams et al., 1994). Lipid-laden myelin debris is taken up and processed by inflammatory cells including neutrophils and macrophages. Myelin then becomes highly concentrated in these phagocytes, persisting in macrophages for weeks after SCI (Greenhalgh and David, 2014; Vargas and Barres, 2007; Wang et al., 2014). In addition, myelin initiates complement-mediated inflammatory pathways with downstream effects on macrophage activation. This review focuses on the myelin-macrophage and complement neuro-immune interactions after SCI. Since myelin is ubiquitously present in the acute and chronically injured spinal cord, we will explore the mechanisms of myelin debris clearance and its downstream inflammatory effects.
Spinal cord injury causes myelin breakdown and immune cell activation
Acute spinal cord trauma ruptures vasculature leading to tissue ischemia and blood brain barrier breakdown and generates neuron and myelin debris. Myelin damage specifically occurs at the time of injury and cellular debris is present in areas of white matter damage within 24hrs (Buss et al., 2005; Ek et al., 2012; Imai et al., 2008). Debris increases within the first week of SCI and persists in the chronically injured spinal cord (Ek et al., 2012; Kozlowski et al., 2014). While damaged myelin is cleared within weeks of peripheral nerve injury, myelin fragments are present for the first year after SCI (Becerra et al., 1995; Vargas and Barres, 2007). Indeed, myelin debris is not cleared from the chronically injured spinal cord until years after injury (Becerra et al., 1995). This time course of acute myelin debris with protracted but eventual removal after SCI occurs across a range mammalian models and in humans (Wang et al., 2014). The sustained presence of myelin debris inhibits axon regeneration, oligodendrocyte differentiation, and remyelination.
Immune cell activation follows a similar time course (Gensel and Zhang, 2015). Neutrophils and complement proteins (discussed below) enter the injury site within the first days after SCI. Microglia are activated at the time of injury with peak activation within the first week of injury. Monocyte-derived macrophages infiltrate the injured spinal cord within days and peak 1–2 weeks after injury. Over time, neutrophils and complement proteins subside but microglia and macrophages persist. Phagocytosis markers are present on these chronically activated cells (Fleming et al., 2006). It is therefore likely that myelin lipids are actively processed by macrophages and are environmental stimuli influencing chronic spinal cord inflammation. Indeed, in areas of Wallerian degeneration, macrophages disappear from the chronically injured spinal cord concomitant with myelin debris clearance (Becerra et al., 1995).
Macrophage receptor-mediated myelin removal
Receptor-mediated phagocytic myelin removal requires binding of damaged myelin to surface receptors and subsequent phagocytosis. These receptors have the potential to bind to myelin directly or to opsonized myelin particles. Both opsonized and non-opsonized myelin removal by macrophages/microglia has been reviewed extensively and will only be mentioned here briefly (Hirata and Kawabuchi, 2002; Neumann et al., 2009; Rotshenker, 2003). Complement-mediated receptor binding, primarily through the pattern recognition receptor (PRR), complement receptor 3 (CR3/CD11b/MAC-1), is a commonly studied mechanism of opsonized myelin clearance. Complement proteins (e.g. complement protein 3) and/or antibodies bind degenerated myelin facilitating CR3 binding and phagocytosis. This process, and the process of non-opsonized myelin clearance, is regulated in part through carbohydrate-lectin receptors including the MAC-2/Galectin-3 receptor among others. The receptor-mediated internalization of myelin, and myelin-receptor binding itself, potentially alters the functional phenotype of the phagocyte. The downstream effects of complement and receptor-mediated myelin removal on macrophage/microglia phenotypes will be discussed below.
It is also well-documented that scavenger receptor AI/II (SRAI/II) participates in macrophage-mediated myelin clearance, for a review see (Rotshenker, 2009). This PRR is capable of binding a variety of lipids and polyanionic ligands. SRAI/II, in combination with CR3, facilitates myelin phagocytosis by microglia and macrophages (da Costa et al., 1997; Reichert and Rotshenker, 2003). Additionally, SRAI/II is implicated as a primary mediator of oxidized lipoprotein uptake in atherosclerosis and leads to the development of foam cells (Greaves and Gordon, 2009). Emerging transcriptional evidence indicates that SCI macrophages increase lipid catabolism after injury and adopt transcription profiles closely resembling foam cells (Zhu et al., 2017). Lipoprotein receptors have been implicated in the foam cell transition but whether SRAI/II facilities this transition in SCI has yet to be determined. This may be challenging considering that SCI results in significant free radical generation and lipid peroxidation (Hall, 2011). It is possible that these oxidative alterations to myelin lipids may alter the receptor pathways through which they are cleared and this caveat is import to consider when modeling myelin clearance in-vitro and interpreting experimental results.
Another potential mechanism of receptor-mediated myelin clearance is through the macrophage receptor with a collagenous structure (MARCO). MARCO is a scavenger receptor related to SRAI/II. Both contain collagenous and scavenger receptor cysteine-rich domains in their extracellular portions giving them similar ligand binding repertoires (Elomaa et al., 1995; Józefowski et al., 2005). Thus, it is likely that MARCO can bind myelin lipids effectively. Although it has not been identified as a myelin receptor in SCI, we recently observed that macrophages upregulate MARCO in response to pro-inflammatory stimuli and express MARCO in the injured spinal cord (Gensel et al., 2017; Orr et al., 2017). MARCO activation, therefore, may be a potential mechanism for pro-inflammatory macrophage-mediated myelin removal in SCI.
Triggering receptor expressed on myeloid cells 2 (TREM2) facilitates microglial phagocytic activity (Takahashi et al., 2005). TREM2 is a sensor for lipid components of damaged myelin and is required for debris clearance in the cuprizone model of demyelination (Daws et al., 2003; Poliani et al., 2015). TREM2 binds polyanionic ligands, such as dextran sulphate, bacterial lipooligosaccharides, and various phospholipids (Cannon et al., 2012). In the case of myelin the likely ligands include phosphatidylethylolamine, phosphotidylserine, and cardiolipin found in myelin membranes (Cannon et al., 2012). Individuals with TREM2 mutations or deficiencies are at higher risk for developing amyotrophic lateral sclerosis, Parkinson’s disease and Alzheimer’s disease (Lill et al., 2015). Notably, individuals with mutations in TREM2 or DAP12, a key-signaling component of TREM2, develop lethal and progressive Nasu-Hakola disease characterized by early onset dementia and demyelinating brain lesions (Paloneva et al., 2010; Verloes et al., 1997). Therefore, TREM2 may be involved in removing damaged myelin following SCI, MS and other conditions. Indeed, microglial TREM2 senses lipid components of myelin debris and is important in regulating transcriptional programs essential for myelin debris clearance (Cantoni et al., 2015; Poliani et al., 2015; Siddiqui et al., 2016). Additional work is needed to examine TREM2 within the context of the myelin-macrophage interactions in SCI. Likewise, TREM2 is part of a large family of TREM and TREM-like receptors with similar ligand binding repertoires that are also unstudied in the context of myelin uptake after SCI (Cannon et al., 2012).
Myelin-Macrophage Interactions
Some of the first studies to examine macrophage-mediated myelin debris clearance in the CNS emerged from the multiple sclerosis (MS) field. Electron microcopy studies revealed myelin debris phagocytosis in active lesions and identified macrophages as facilitators of continued demyelination (Prineas, 1975; Prineas and Connell, 1978). These catalyzed further studies largely concerned with the roles of anti-myelin antibodies, complement, and other pathways in relation to the chronic demyelination observed in MS. A 1994 publication by Williams et al. in the Journal of Neuroscience Research identified an inflammatory role for myelin debris (Williams et al., 1994). Specifically, they reported microglial activation with increased pro-inflammatory cytokine and ROS production with myelin phagocytosis (Williams et al., 1994). These observations indicate that myelin debris may be an inflammatory stimulus for pro-inflammatory microglia/macrophage activation.
In 1990’s, work by Brück and Friede and van der Laan et al. demonstrated the importance of macrophage CR3 in mediating myelin uptake. They observed that myelin induced production of tumor necrosis factor-alpha (TNF-α) and nitric oxide (van der Laan et al., 1996). These effects were enhanced through complement opsonization of myelin and blocked through antibody-mediated-inhibition of CR3 (Brück and Friede, 1991; 1990; van der Laan et al., 1996). This work is supported by a more recent study in an SCI model which reported myelin invoked pro-inflammatory macrophage responses in vivo (Sun et al., 2010). In that paper, CR3-mediated uptake of myelin and downstream activation of FAK/PI3K/Akt/NF-κβ signaling pathways increased pro-inflammatory, M1-like cytokine release and decreased M2 cytokine release (Sun et al., 2010). Collectively, these data highlight that receptor-mediated myelin removal can alter macrophage phenotypes and that removal through CR3 drives pro-inflammatory macrophage activation in SCI.
While these studies implicate myelin as a pro-inflammatory macrophage stimulus in SCI, the role of myelin and lipid processing on M1/M2 macrophage polarization in vivo is controversial. Specifically, myelin-laden “foamy” cells express a wide variety of anti-inflammatory molecules with some intermediate M1–M2 phenotypes in MS (Boven et al., 2006; Vogel et al., 2013). In arthrosclerosis, foam cell formation is associated with a downregulation of pro-inflammatory gene expression (Spann et al., 2012). In contrast, in SCI, foam cell formation and macrophage lipid accumulation is associated with decreased M2 activation (Wang et al., 2014). Further, M1 macrophage polarization predominates in SCI despite the large presence of myelin debris (Kroner et al., 2014). Interestingly, the relative expression of pro- and anti-inflammatory markers appears to depend on the lesion or tissue microenvironment. For example, in active MS lesions, anti-inflammatory marker expression predominates on foam cell macrophages in the lesion center and inner rim while pro-inflammatory marker expression is more widespread (Boven et al., 2006; Vogel et al., 2013). Similarly, a more recent evaluation of foam cells within the context of the M1/M2 macrophage paradigm observed a full range of M1–M2 foam cell activation states depending upon the microenvironments from which the cells were isolated (Thomas et al., 2015a; 2015b). In the case of SCI, two environmental cues that may promote M1 polarization in the presence of myelin are TNF and intracellular iron, perhaps reflective of the increased hemorrhage in SCI lesions relative to those in MS (Kroner et al., 2014).
To better elucidate the role myelin plays in macrophage polarization, researchers often stimulate macrophages with myelin in vitro. However, despite using fairly similar stimulation paradigms, there are contrasting reports that implicate myelin as both a pro-inflammatory and anti-inflammatory stimulus. To better understand how to reconcile these conflicting reports, we examined the myelin stimulations in detail. A few common themes emerged that may account for the conflicting results. First, in several of these in-vitro paradigms, researchers load macrophages with myelin prior to applying inflammatory stimuli. In response to pro-inflammatory stimuli, these myelin-laden macrophages almost invariably express anti-inflammatory mediators and/or stop responding to the pro-inflammatory stimuli (Bogie et al., 2013; 2012) (Boven et al., 2006). Second, myelin stimulation in isolation invokes either no phenotypic activation or causes release of reactive oxygen species and pro-inflammatory cytokines with a few reports of subtle M2-like activation in a cell-type specific manner (Kroner et al., 2014; Sun et al., 2010; van der Laan et al., 1996; van Rossum et al., 2008; Wang et al., 2014; Williams et al., 1994). Third, when myelin is delivered to macrophages already stimulated to be either M1 or M2, or when myelin and other stimuli are presented at the same time, a range of responses has been reported. For example, pre-stimulation with M1 or M2 stimuli results in a myelin-induced potentiation of M1 activation (Siddiqui et al., 2016; Wang et al., 2014). Myelin co-administration with pro-inflammatory stimuli can invoke further M1 activation albeit in a time-dependent manner with increased M2 activation over time (Y. Liu et al., 2006). A subtlety distinct protocol added myelin to M1 polarized macrophages following the removal of the inflammatory stimuli and observed anti-inflammatory effects (Kroner et al., 2014). Notably, in that study, if pro-inflammatory stimuli are present during myelin activation, M1 polarization was observed (Kroner et al., 2014). It is also important to note that researchers utilize various cell types including bone marrow derived macrophages, primary microglia, cell lines, peritoneal macrophages, and blood monocytes among others for in vitro models. All of these cell types have subtle differences in their basal activation state and their ability to take up and respond to myelin (Durafourt et al., 2012; van Rossum et al., 2008). Collectively, these myelin-macrophage studies in vitro indicate that: 1) myelin dampens the macrophage response to subsequent stimuli; 2) in isolation myelin may act as a pro-inflammatory stimulus that drives M1 type-activation; and 3) when combined with other stimuli, initially myelin facilitates M1 polarization but this response varies over time.
The results of these in vitro studies indicate that myelin is capable of producing downstream effects on macrophage that vary under different cellular and environmental conditions. This highlights the impact that stimulation type and timing, relative to myelin application, has on myelin-induced shifts in macrophage phenotype. Microglia and macrophages in the injured spinal cord would likely be exposed to inflammatory stimuli before or concurrent with the clearance of myelin debris. Macrophages also encounter distinct stimuli in the SCI vs. MS lesion environment. It is therefore possible that different macrophage activation states during myelin processing leads to varied inflammatory responses in SCI and MS. Until the molecular mechanisms through which myelin exerts its effects are better understood, it remains unclear how myelin and inflammatory stimuli synergize to produce different cellular responses.
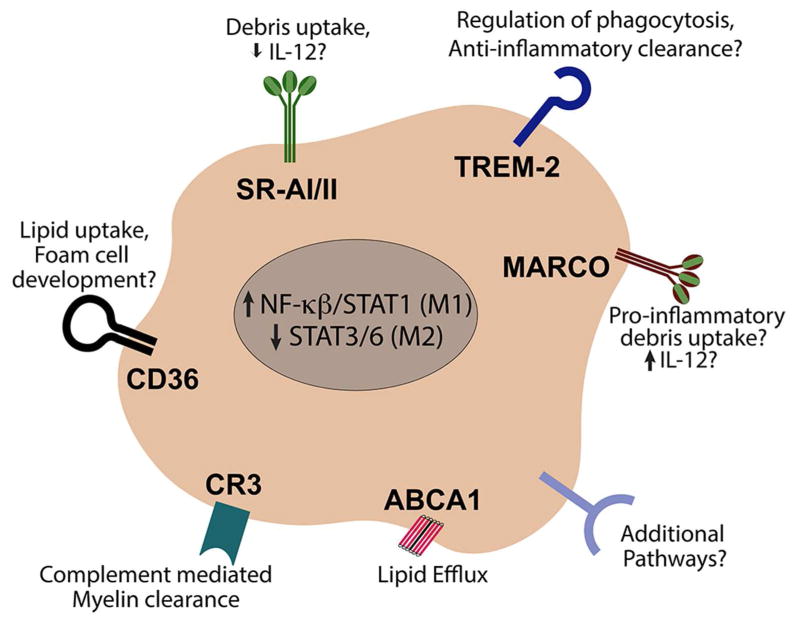
In addition, the macrophage response to myelin uptake varies depending upon the receptors mediating phagocytosis (Figure 1). In traditional responses to infection and damage, macrophage PRRs often share redundancies in the molecular structures they target. It has been proposed that collective engagement of different PRRs can influence the cellular response to an inflammatory insult (Józefowski et al., 2005; Mukhopadhyay et al., 2004). Indeed, in SCI, the collaborative engagement of PRRs vs. activation in isolation, yields distinct reparative or pathological functions (Gensel et al., 2015). Notably, the PRRs capable of recognizing and clearing myelin, as discussed above, appear to mediate contrasting cellular responses along the spectrum of macrophage activation states. This suggests that the specific combination of myelin clearance receptors utilized after injury may influence the inflammatory effects of myelin debris. For example, in-vitro, MARCO is important in mediating pro-inflammatory IL-12 production in response to inflammatory stimuli (Józefowski et al., 2005). Similarly, it is well documented that macrophages increase IL-12 release with myelin stimulation (Sun et al., 2010; Wang et al., 2014). Given that MARCO is present on macrophages after SCI (Gensel et al., 2017), it is possible that MARCO-mediated myelin clearance may influence the M1-like polarization observed in SCI. Conversely, SRAI/II has been implicated in the inhibition of IL-12 production (Józefowski et al., 2005; Józefowski and Kobzik, 2004). Whether the differential function and regulation of these macrophage receptors are of similar importance in the context of myelin debris clearance after SCI is unknown (Figure 1).
Figure 1. Macrophage receptors potentially mediating myelin clearance and inflammatory activity after spinal cord injury.
In addition to activating a number of different receptors, in the context of SCI, myelin also drives downstream pathways (Stat1) associated with pro-inflammatory (M1) macrophage activation. References can be found in the main text.
Following SCI, or other neurological conditions, myelin clearance is likely influenced simultaneously by multiple receptors. This receptor cross-talk could be additive, synergistic, or antagonistic in terms of intracellular signaling depending on which receptors are present and activated during clearance (Lee and Kim, 2007; Natarajan et al., 2006). Interestingly, certain bacterial strains have evolved to manipulate this PRR crosstalk by CR3, MARCO, TLRs, and C5aR (discussed below) to block the production of IL-12 and IFN-γ thereby impairing macrophage bacterial clearance (Hajishengallis and Lambris, 2011). Whether a similar approach could be harnessed to manipulate myelin mediated inflammatory responses after SCI has yet to be studied.
Additionally, TREM2 plays a role in myelin clearance and integrity. As discussed above, it is important in regulating the transcriptional programs essential for myelin phagocytosis (Cantoni et al., 2015; Poliani et al., 2015; Siddiqui et al., 2016). Further, TREM2 signaling facilitates the production of tropic factors important in oligodendrocyte differentiation, survival, and remyelination (Poliani et al., 2015). Interestingly, overexpression of TREM2 increases the efficiency of non-inflammatory phagocytosis (Takahashi et al., 2005; 2007). TREM2 overexpression in macrophages also induces an M2 phenotype and M2 activation is inhibited in TREM2 KO macrophages (Seno et al., 2009; Takahashi et al., 2005). In an experimental model of MS, TREM2 facilitated myelin removal while increasing anti-inflammatory cytokine production (Takahashi et al., 2007). Whether TREM2 drives M2 macrophage activation is the context of SCI is unknown but these studies suggest that it may be a pro-reparative pathway for myelin debris clearance.
CD36 (a class B scavenger receptor) is also implicated in macrophage-mediated myelin clearance after SCI. Recently, Zhu et al., 2017, examined shifts in macrophage transcriptional profiles from 3 to 7 days post injury (dpi). They observed a significant shift from genes controlling cytokine signaling and cellular migration at 3dpi to a profile dominated by lipid catabolism at 7dpi through the liver X and retinoid X receptor (LXR/RXR) and peroxisome proliferator-activated receptors (PPAR)/RXR canonical pathways (Zhu et al., 2017). Further, they targeted the most enriched lipid receptor identified, CD36, and found that its genetic deletion reduced lipid accumulation in macrophages and improved functional outcomes after SCI (Zhu et al., 2017). Interestingly, the RXR signaling pathways are similar to those activated by foam cells within atherosclerotic lesions.
Independent of the receptor mediating myelin clearance, a previous study of foamy macrophages in SCI showed that myelin stimulation shifted the balance of macrophage activation towards M1 activation. Specifically, myelin stimulation increased pro-inflammatory NF-κβ/STAT1 signaling and decreased M2-associated STAT3/STAT6 signaling (Wang et al., 2014). Further, while myelin increased lipid efflux and activated ATP-binding cassette transporter A1 (ABCA1) in macrophages, in foamy cells, myelin decreased the phagocytic capacity for necrotic neutrophils (Wang et al., 2014). Since foamy macrophages are present in SCI, they proposed that these spent, but non-phagocytosed, neutrophils released toxins and contribute to secondary injury after SCI. This is a novel potential mechanism through which foam-like, myelin-laden macrophages may contribute to secondary injury processes (Wang et al., 2014). Given the extensive lipid debris accumulating within SCI macrophages, additional comparisons to foam cells and atherosclerosis may lead to new therapeutic targets.
Another important factor that likely regulates the myelin-macrophage neuro-immune interaction in SCI is the phagocytic cell origin. Microglia have the phagocytic capacity to remove damage myelin and are the predominant phagocyte to clear debris in the acutely injured spinal cord (Greenhalgh and David, 2014). However, by three days post injury, this role is largely taken over by infiltrating macrophages (Greenhalgh and David, 2014). Specifically, in the first weeks after SCI, MAC-2 positive bone marrow-derived macrophages enter the lesion site and are positive for myelin debris (Wang et al., 2014). These infiltrating macrophages accumulate in the lesion over time and contain lipid debris for at least 42dpi (Greenhalgh and David, 2014). In contrast, CX3CR1high resident microglia are primarily found along the periphery of the lesion/lipid plaque in areas of less concentrated myelin debris (Wang et al., 2014). The downstream effects of this differential distribution of macrophages and microglia relative to lipid debris has been discussed previously (Zhou et al., 2014) but it is interesting to consider how cell-specific receptor expression may mediate myelin polarization in SCI. For example, MARCO is primarily expressed by infiltrating and not resident myeloid cells (Getts et al., 2014). Similarly, MAC-2 expression is specific to monocyte derived macrophages after SCI (Wang et al., 2014). TREM2, however, is predominantly expressed by microglia (Schmid et al., 2002). As discussed above, these receptors have different propensities for pro-inflammatory or anti-inflammatory cytokine release in response to myelin stimulation (Figure 1). It is therefore possible that varied receptor expression on microglia and monocyte-derived macrophages predisposes these cells for different inflammatory responses to SCI myelin debris. Determining the relative contribution of microglia vs. macrophages on myelin-mediated SCI inflammation will be challenging, however, based upon the observation that macrophage myelin phagocytosis varies depending upon the inflammatory cues driving recruitment to the site of injury (Slobodov et al., 2001).
Relative to the injured spinal cord, myelin may induce deleterious pro-inflammatory macrophage activation (Kroner et al., 2014; Wang et al., 2014). Myelin and myelin phagocytosis likely potentiate signaling pathways and polarization states in macrophage and function as direct inflammatory stimuli (Figure 1). Determining the mechanisms and environmental conditions through which myelin can induce these effects in immune cells could lead to novel therapies for SCI and other neuroinflammatory disorders.
Complement mediated myelin clearance
In addition to macrophages, the complement system is a key inflammatory mediator of myelin debris removal. The detailed pathways and diverse roles of complement have been reviewed in the context of SCI (Peterson and Anderson, 2014). Here we discuss the contributions of complement to myelin clearance and how this may influence inflammatory pathways after SCI. Within the complement system there are numerous proteins in the plasma that enzymatically mark pathogens for destruction. Specifically, proteins of the complement cascade selectively recognize pathogen associated molecular patterns (PAMPs) or damaged associated molecular patterns (DAMPs) and opsonize, or tag, these PAMPs or DAMPs for removal. Complement component 3 proteins, most notably C3b, act as opsonizing agents. Opsonized pathogens are targets for removal through complement receptors on phagocytes (such as CR3 discussed above) or through secondary complement pathways. Regardless of which complement pathway is initiated (classical, alternative, or lectin), a secondary complement membrane attack complex is formed creating large holes in the target membrane and ultimately causing pathogen lysis (Parham, 2009). Complement proteins opsonize myelin debris in the CNS and thus the complement system is a critical initiator of myelin invoked inflammatory responses to SCI in addition to its other direct functions (Peterson and Anderson, 2014; Sun et al., 2010; van der Laan et al., 1996). Further, the activation of this immune pathway through myelin interactions, DAMPs, or other means can directly damage intact myelin, oligodendrocytes, and neurons thereby driving inflammation by increasing myelin and cellular debris or other inflammatory mediators.
The primary source of complement is the liver. It produces substantial quantities of inactive complement. This complement is stored in the plasma until it is activated in response to infection or damage. After SCI, disruption of the blood brain barrier allows complement proteins to enter the lesion site. Indeed, complement increases in the injured spinal cord within 1 day of SCI in both rats and humans (Nguyen et al., 2008). SCI-induced inflammatory cytokines may also increase complement serum levels at these acute time points (Rebhun and Botvin, 1980). While plasma derived complement is likely the main initiator of early myelin clearance after SCI, complement is present in the chronically injured spinal cord (Anderson et al., 2004). Many cells of the immune and nervous systems are capable of producing complement proteins including macrophages/monocytes, lymphocytes, and neutrophils, all of which enter the lesion after SCI, and resident astrocytes, neurons, and microglia within the CNS (Barnum, 1995; Beck et al., 2010; Peterson and Anderson, 2014). These endogenous sources of complement in the SCI microenvironment are largely unstudied but could be critical in complement mediated reactions and myelin clearance after injury.
Many factors within the SCI lesion environment potentially activate the complement cascade with downstream effects on myelin clearance (Figure 2). For example, binding of complement protein C1q to oligodendrocyte myelin glycoprotein (OMgp) drives myelin opsonization and clearance (Johns and Bernard, 1997). OMgp contains an amino acid motif that shares homology with C1q binding sites on PAMPs/DAMPs (Johns and Bernard, 1997). OMgp and C1q levels increase after SCI (Anderson et al., 2004; Dou et al., 2009). Since C1q binding to OMgp initiates complement activation, it is likely that C1q mediates myelin debris clearance and inflammation after SCI (Figure 2). Indeed, C1q knockout mice have improved SCI recovery and tissue sparing and altered macrophage activation compared to wild-type SCI controls (Galvan et al., 2008).
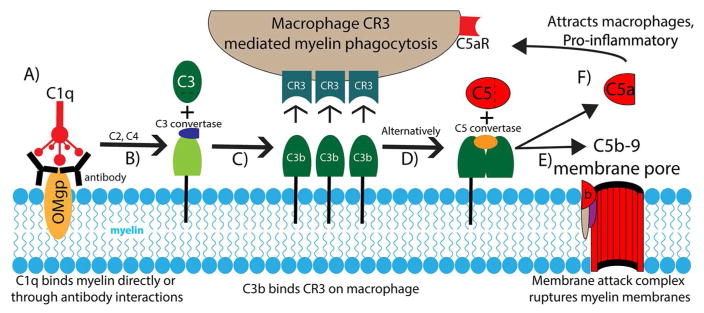
Figure 2. Complement system in myelin clearance and macrophage recruitment after spinal cord injury.
Numerous complement pathways are initiated by myelin debris after SCI. (A) C1q binds directly to myelin oligodendrocyte myelin glycoprotein (OMgp) or to an antibody intermediate as depicted. (B) C1q binding initiates the formation of a C3 convertase capable of cleaving C3. (C) The C3b cleavage fragment opsonizes myelin debris and binds to macrophage complement receptor 3 (CR3) initiating the phagocytosis of myelin debris. (D) C3b can also induce the formation of a C5 convertase. (E) The C5 convertase initiates the cleavage of C5 and the subsequent recruitment of C6-9 creating the C5b-9 membrane attack complex capable of rupturing myelin membrane debris or intact oligodendrocytes. (F) The C5a fragment released during this process acts as both a potent recruitment signal for macrophages to the site of complement opsonization, through the C5 receptor (C5aR) and as a pro-inflammatory, vasoactive stimulus on a variety of other cell types. References can be found in the main text.
Another potentially important complement protein in myelin-immune interactions after SCI is C5. Studies conducted primarily in models of MS and Alzheimer’s disease demonstrate that formation of the C5b-9 membrane attack complex on oligodendrocytes leads to extensive demyelination and oligodendrocyte cell death (Bradt et al., 1998; W. T. Liu et al., 1983; Rus et al., 2006). This lytic attack requires the proteolytic cleavage of complement into bioactive proteins including C5a among others (Figure 2). While not well understood within the CNS, in the periphery C5a binds to receptors on mast cells and basophils ultimately inducing degranulation and release of vasoactive substances. These substances, such as histamine, increase blood vessel permeability (Parham, 2009). Further, C5a is a potent chemoattractant for monocytes and neutrophils (Marder et al., 1985). C5a can therefore drive inflammation by increasing recruitment and efflux of immune cells from the blood to sites of cellular debris (Miller and Stella, 2009). In the context of myelin debris induced complement activation after SCI, increased C5a could act as an ongoing recruitment signal and mediator of macrophages entry into injury site. A recent study demonstrated that loss of C5aR, the receptor for C5a, reduces macrophage recruitment and inflammatory cytokine production early after SCI (Brennan et al., 2015). Further, C5a has direct apoptotic effects on neurons that may contribute to secondary injury thereby increasing cellular debris (Farkas et al., 1998; Humayun et al., 2009). It is therefore feasible that complement-mediated myelin debris clearance results in pro-inflammatory macrophage activation, increased demyelination, and further complement activation (Figure 2). Collectively, complement, myelin, and macrophage interactions may form a positive feedback cycle maintaining a chronic inflammatory state in the injured spinal cord.
Conclusions/Summary
Spinal cord injuries and other neuroinflammatory conditions inevitably result in tissue destruction and generation of cellular and myelin debris. While debris must be cleared to begin recovery, it has direct effects on the cells that clear it, namely macrophages and microglia. The mechanisms contributing to the failed debris clearance in SCI by phagocytes remain unclear, as do the myelin-mediated pathways invoking inflammatory responses including myelin-driven complement mediated inflammation. Myelin is not inherently pro-inflammatory in all scenarios but is capable of producing detrimental outcomes when cleared and processed under specific cellular and environmental conditions. This indicates that the pathological effects of myelin may be receptor or activation state dependent. Targeting these pathways and receptors opens the possibility for therapeutic interventions to improve recovery after SCI. Further, given myelin’s ubiquitous presence in the CNS, the development of new therapies will likely impact a variety of disorders in which complement, myelin, and macrophage interactions contribute to persistent inflammation.
Significance Statement.
Myelin debris and chronic inflammation are hallmarks of central nervous system pathology. Understanding the neuro-immune interactions between myelin and inflammatory mediators such as macrophages and complement has the potential to uncover novel therapeutic targets applicable for a range of central nervous system disorders.
Acknowledgments
This work is supported by NIH NINDS R01 NS091582 and the Spinal Cord and Brain Injury Research Center at the University of Kentucky.
Footnotes
Conflict of Interest: The authors have no actual or potential conflicts of interest.
Role of Authors: Both Timothy J. Kopper and John C. Gensel conceived of the content and wrote the manuscript.
References
- Anderson AJ, Robert S, Huang W, Young W, Cotman CW. Activation of complement pathways after contusion-induced spinal cord injury. J Neurotrauma. 2004;21:1831–1846. doi: 10.1089/neu.2004.21.1831. [DOI] [PubMed] [Google Scholar]
- Barnum SR. Complement biosynthesis in the central nervous system. Crit Rev Oral Biol Med. 1995;6:132–146. doi: 10.1177/10454411950060020301. [DOI] [PubMed] [Google Scholar]
- Becerra JL, Puckett WR, Hiester ED, Quencer RM, Marcillo AE, Post MJ, Bunge RP. MR-pathologic comparisons of wallerian degeneration in spinal cord injury. AJNR Am J Neuroradiol. 1995;16:125–133. [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogie JFJ, Jorissen W, Mailleux J, Nijland PG, Zelcer N, Vanmierlo T, van Horssen J, Stinissen P, Hellings N, Hendriks JJA. Myelin alters the inflammatory phenotype of macrophages by activating PPARs. Acta Neuropathol Commun. 2013;1:43. doi: 10.1186/2051-5960-1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogie JFJ, Timmermans S, Huynh-Thu VA, Irrthum A, Smeets HJM, Gustafsson J-Å, Steffensen KR, Mulder M, Stinissen P, Hellings N, Hendriks JJA. Myelin-derived lipids modulate macrophage activity by liver X receptor activation. PLoS ONE. 2012;7:e44998. doi: 10.1371/journal.pone.0044998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129:517–526. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. J Exp Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FH, Gordon R, Lao HW, Biggins PJ, Taylor SM, Franklin RJM, Woodruff TM, Ruitenberg MJ. The Complement Receptor C5aR Controls Acute Inflammation and Astrogliosis following Spinal Cord Injury. Journal of Neuroscience. 2015;35:6517–6531. doi: 10.1523/JNEUROSCI.5218-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W, Friede RL. The role of complement in myelin phagocytosis during PNS wallerian degeneration. J Neurol Sci. 1991;103:182–187. doi: 10.1016/0022-510x(91)90162-z. [DOI] [PubMed] [Google Scholar]
- Brück W, Friede RL. Anti-macrophage CR3 antibody blocks myelin phagocytosis by macrophages in vitro. Acta Neuropathol. 1990;80:415–418. doi: 10.1007/BF00307696. [DOI] [PubMed] [Google Scholar]
- Buss A, Pech K, Merkler D, Kakulas BA, Martin D, Schoenen J, Noth J, Schwab ME, Brook GA. Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain. 2005;128:356–364. doi: 10.1093/brain/awh355. [DOI] [PubMed] [Google Scholar]
- Cannon JP, O’Driscoll M, Litman GW. Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics. 2012;64:39–47. doi: 10.1007/s00251-011-0562-4. [DOI] [PubMed] [Google Scholar]
- Cantoni C, Bollman B, Licastro D, Xie M, Mikesell R, Schmidt R, Yuede CM, Galimberti D, Olivecrona G, Klein RS, Cross AH, Otero K, Piccio L. TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 2015;129:429–447. doi: 10.1007/s00401-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa CC, van der Laan LJ, Dijkstra CD, Brück W. The role of the mouse macrophage scavenger receptor in myelin phagocytosis. Eur J Neurosci. 1997;9:2650–2657. doi: 10.1111/j.1460-9568.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171:594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- Dou F, Huang L, Yu P, Zhu H, Wang X, Zou J, Lu P, Xu XM. Temporospatial expression and cellular localization of oligodendrocyte myelin glycoprotein (OMgp) after traumatic spinal cord injury in adult rats. J Neurotrauma. 2009;26:2299–2311. doi: 10.1089/neu.2009.0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- Ek CJ, Habgood MD, Dennis R, Dziegielewska KM, Mallard C, Wheaton B, Saunders NR. Pathological changes in the white matter after spinal contusion injury in the rat. PLoS ONE. 2012;7:e43484. doi: 10.1371/journal.pone.0043484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- Farkas I, Baranyi L, Liposits ZS, Yamamoto T, Okada H. Complement C5a anaphylatoxin fragment causes apoptosis in TGW neuroblastoma cells. Neuroscience. 1998;86:903–911. doi: 10.1016/s0306-4522(98)00108-0. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Galvan MD, Luchetti S, Burgos AM, Nguyen HX, Hooshmand MJ, Hamers FPT, Anderson AJ. Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. Journal of Neuroscience. 2008;28:13876–13888. doi: 10.1523/JNEUROSCI.2823-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep. 2017;7:40144. doi: 10.1038/srep40144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Wang Y, Guan Z, Beckwith KA, Braun KJ, Wei P, Mctigue DM, Popovich PG. Toll-Like Receptors and Dectin-1, a C-Type Lectin Receptor, Trigger Divergent Functions in CNS Macrophages. Journal of Neuroscience. 2015;35:9966–9976. doi: 10.1523/JNEUROSCI.0337-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kühn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJC. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6:219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res. 2009;50(Suppl):S282–6. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. Journal of Neuroscience. 2014;34:6316–6322. doi: 10.1523/JNEUROSCI.4912-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED. Antioxidant Therapies for Acute Spinal Cord Injury. Neurotherapeutics. 2011;8:152–167. doi: 10.1007/s13311-011-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Kawabuchi M. Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration. Microsc Res Tech. 2002;57:541–547. doi: 10.1002/jemt.10108. [DOI] [PubMed] [Google Scholar]
- Humayun S, Gohar M, Volkening K, Moisse K, Leystra-Lantz C, Mepham J, McLean J, Strong MJ. The complement factor C5a receptor is upregulated in NFL−/− mouse motor neurons. J Neuroimmunol. 2009;210:52–62. doi: 10.1016/j.jneuroim.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Imai M, Watanabe M, Suyama K, Osada T, Sakai D, Kawada H, Matsumae M, Mochida J. Delayed accumulation of activated macrophages and inhibition of remyelination after spinal cord injury in an adult rodent model. J Neurosurg Spine. 2008;8:58–66. doi: 10.3171/SPI-08/01/058. [DOI] [PubMed] [Google Scholar]
- Johns TG, Bernard CC. Binding of complement component Clq to myelin oligodendrocyte glycoprotein: a novel mechanism for regulating CNS inflammation. Molecular Immunology. 1997;34:33–38. doi: 10.1016/s0161-5890(97)00005-9. [DOI] [PubMed] [Google Scholar]
- Józefowski S, Arredouani M, Sulahian T, Kobzik L. Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J Immunol. 2005;175:8032–8041. doi: 10.4049/jimmunol.175.12.8032. [DOI] [PubMed] [Google Scholar]
- Józefowski S, Kobzik L. Scavenger receptor A mediates H2O2 production and suppression of IL-12 release in murine macrophages. J Leukoc Biol. 2004;76:1066–1074. doi: 10.1189/jlb.0504270. [DOI] [PubMed] [Google Scholar]
- Kozlowski P, Rosicka P, Liu J, Yung AC, Tetzlaff W. In vivo longitudinal Myelin Water Imaging in rat spinal cord following dorsal column transection injury. Magn Reson Imaging. 2014;32:250–258. doi: 10.1016/j.mri.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and Increased Intracellular Iron Alter Macrophage Polarization to a Detrimental M1 Phenotype in the Injured Spinal Cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim Y-J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- Lill CM, Rengmark A, Pihlstrøm L, Fogh I, Shatunov A, Sleiman PM, Wang LS, Liu T, Lassen CF, Meissner E, Alexopoulos P, Calvo A, Chio A, Dizdar N, Faltraco F, Forsgren L, Kirchheiner J, Kurz A, Larsen JP, Liebsch M, Linder J, Morrison KE, Nissbrandt H, Otto M, Pahnke J, Partch A, Restagno G, Rujescu D, Schnack C, Shaw CE, Shaw PJ, Tumani H, Tysnes OB, Valladares O, Silani V, van den Berg LH, van Rheenen W, Veldink JH, Lindenberger U, Steinhagen-Thiessen E, Teipel S, Perneczky R, Hakonarson H, Hampel H, von Arnim CAF, Olsen JH, Van Deerlin VM, Al-Chalabi A, Toft M, Ritz B, Bertram L SLAGEN Consortium. The role of TREM2 R47H as a risk factor for Alzheimer“s disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson”s disease. Alzheimers Dement. 2015;11:1407–1416. doi: 10.1016/j.jalz.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WT, Vanguri P, Shin ML. Studies on demyelination in vitro: the requirement of membrane attack components of the complement system. J Immunol. 1983;131:778–782. [PubMed] [Google Scholar]
- Liu Y, Hao W, Letiembre M, Walter S, Kulanga M, Neumann H, Fassbender K. Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species. Journal of Neuroscience. 2006;26:12904–12913. doi: 10.1523/JNEUROSCI.2531-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Chenoweth DE, Goldstein IM, Perez HD. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985;134:3325–3331. [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Miller AM, Stella N. Microglial cell migration stimulated by ATP and C5a involve distinct molecular mechanisms: quantification of migration by a novel near-infrared method. Glia. 2009;57:875–883. doi: 10.1002/glia.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Herre J, Brown GD, Gordon S. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology. 2004;112:521–530. doi: 10.1111/j.1365-2567.2004.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan M, Lin KM, Hsueh RC, Sternweis PC, Ranganathan R. A global analysis of cross-talk in a mammalian cellular signalling network. Nat Cell Biol. 2006;8:571–580. doi: 10.1038/ncb1418. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJM. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HX, Galvan MD, Anderson AJ. Characterization of early and terminal complement proteins associated with polymorphonuclear leukocytes in vitro and in vivo after spinal cord injury. J Neuroinflammation. 2008;5:26. doi: 10.1186/1742-2094-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MB, Simkin J, Bailey WM, Kadambi NH, McVicar AL, Veldhorst AK, Gensel J. Compression Decreases Anatomical and Functional Recovery and Alters Inflammation after Contusive Spinal Cord Injury. J Neurotrauma. 2017 doi: 10.1089/neu.2016.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloneva J, Autti T, Hakola P, Haltia MJ. Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL) 2010 [PubMed] [Google Scholar]
- Parham P. The immune system. 3. Taylor & Francis; 2009. [Google Scholar]
- Peterson SL, Anderson AJ. Complement and spinal cord injury: traditional and non-traditional aspects of complement cascade function in the injured spinal cord microenvironment. Exp Neurol. 2014;258:35–47. doi: 10.1016/j.expneurol.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Poliani PL, Wang Y, Fontana E, Robinette ML, Yamanishi Y, Gilfillan S, Colonna M. TREM2 sustains microglial expansion during aging and response to demyelination. J Clin Invest. 2015;125:2161–2170. doi: 10.1172/JCI77983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas J. Pathology of the early lesion in multiple sclerosis. Hum Pathol. 1975;6:531–554. doi: 10.1016/s0046-8177(75)80040-2. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Connell F. The fine structure of chronically active multiple sclerosis plaques. Neurology. 1978;28:68–75. doi: 10.1212/wnl.28.9_part_2.68. [DOI] [PubMed] [Google Scholar]
- Rebhun J, Botvin J. Complement elevation in spinal cord injury. Ann Allergy. 1980;44:287–288. [PubMed] [Google Scholar]
- Reichert F, Rotshenker S. Complement-receptor-3 and scavenger-receptor-AI/II mediated myelin phagocytosis in microglia and macrophages. Neurobiol Dis. 2003;12:65–72. doi: 10.1016/S0969-9961(02)00008-6. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. The role of Galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J Mol Neurosci. 2009;39:99–103. doi: 10.1007/s12031-009-9186-7. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Microglia and macrophage activation and the regulation of complement-receptor-3 (CR3/MAC-1)-mediated myelin phagocytosis in injury and disease. J Mol Neurosci. 2003;21:65–72. doi: 10.1385/JMN:21:1:65. [DOI] [PubMed] [Google Scholar]
- Rus H, Cudrici C, David S, Niculescu F. The complement system in central nervous system diseases. Autoimmunity. 2006;39:395–402. doi: 10.1080/08916930600739605. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TA, Lively S, Schlichter LC. Complex molecular and functional outcomes of single versus sequential cytokine stimulation of rat microglia. J Neuroinflammation. 2016;13:66. doi: 10.1186/s12974-016-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodov U, Reichert F, Mirski R, Rotshenker S. Distinct inflammatory stimuli induce different patterns of myelin phagocytosis and degradation in recruited macrophages. Exp Neurol. 2001;167:401–409. doi: 10.1006/exnr.2000.7559. [DOI] [PubMed] [Google Scholar]
- Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CRH, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wang X, Chen T, Li T, Cao K, Lu A, Chen Y, Sun D, Luo J, Fan J, Young W, Ren Y. Myelin activates FAK/Akt/NF-kappaB pathways and provokes CR3-dependent inflammatory response in murine system. PLoS ONE. 2010;5:e9380. doi: 10.1371/journal.pone.0009380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford CDP, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AC, Eijgelaar WJ, Daemen MJAP, Newby AC. The pro-fibrotic and anti-inflammatory foam cell macrophage paradox. Genomics Data. 2015a;6:136–138. doi: 10.1016/j.gdata.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AC, Eijgelaar WJ, Daemen MJAP, Newby AC. Foam Cell Formation In Vivo Converts Macrophages to a Pro-Fibrotic Phenotype. PLoS ONE. 2015b;10:e0128163. doi: 10.1371/journal.pone.0128163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan LJ, Ruuls SR, Weber KS, Lodder IJ, Döpp EA, Dijkstra CD. Macrophage phagocytosis of myelin in vitro determined by flow cytometry: phagocytosis is mediated by CR3 and induces production of tumor necrosis factor-alpha and nitric oxide. J Neuroimmunol. 1996;70:145–152. doi: 10.1016/S0165-5728(96)00110-5. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hilbert S, Straßenburg S, Hanisch UK, Brück W. Myelin-phagocytosing macrophages in isolated sciatic and optic nerves reveal a unique reactive phenotype. Glia. 2008;56:271–283. doi: 10.1002/glia.20611. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Verloes A, Maquet P, Sadzot B, Vivario M, Thiry A, Franck G. Nasu-Hakola syndrome: polycystic lipomembranous osteodysplasia with sclerosing leucoencephalopathy and presenile dementia. J Med Genet. 1997;34:753–757. doi: 10.1136/jmg.34.9.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel DYS, Vereyken EJF, Glim JE, Heijnen PDAM, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun L, Guo L, Bai P, Sun D, Fan J, He X, Young W, Ren Y. Macrophages in spinal cord injury: Phenotypic and functional change from exposure to myelin debris. Glia. 2014 doi: 10.1002/glia.22774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Ulvestad E, Waage A, Antel JP, McLaurin J. Activation of adult human derived microglia by myelin phagocytosis in vitro. J Neurosci Res. 1994;38:433–443. doi: 10.1002/jnr.490380409. [DOI] [PubMed] [Google Scholar]
- Zhou X, He X, Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res. 2014;9:1787–1795. doi: 10.4103/1673-5374.143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Lyapichev K, Lee DH, Motti D, Ferraro NM, Zhang Y, Yahn S, Soderblom C, Zha J, Bethea JR, Spiller KL, Lemmon VP, Lee JK. Macrophage Transcriptional Profile Identifies Lipid Catabolic Pathways That Can Be Therapeutically Targeted after Spinal Cord Injury. Journal of Neuroscience. 2017;37:2362–2376. doi: 10.1523/JNEUROSCI.2751-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]