Fig. 1.
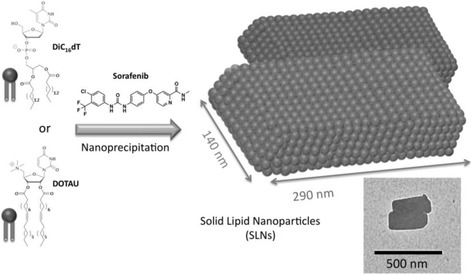
Scheme of SLNs formulation. Chemical structures of an anionic nucleotide-lipid, the thymidine 3′-(1,2-dipalmitoyl-sn-glycero-3-phosphate) (diC16dT), a cationic-nucleoside-lipid DOTAU (2′,3′-dioleyl-5′-deoxy-5′-trimethyl-ammonium-uridine), and sorafenib used in this study (left). Schematic drawing of SLNs with parallelepiped shapes obtained after nanoprecipitation of a nucleolipid (either diC16dT or DOTAU, leading to SLN− and SLN+, respectively) with sorafenib (right). The schematic representation is adapted from the transmission electronic microscopy (TEM) image showing DOTAU sorafenib-loaded nanoparticles (inset, bar 500 nm)
