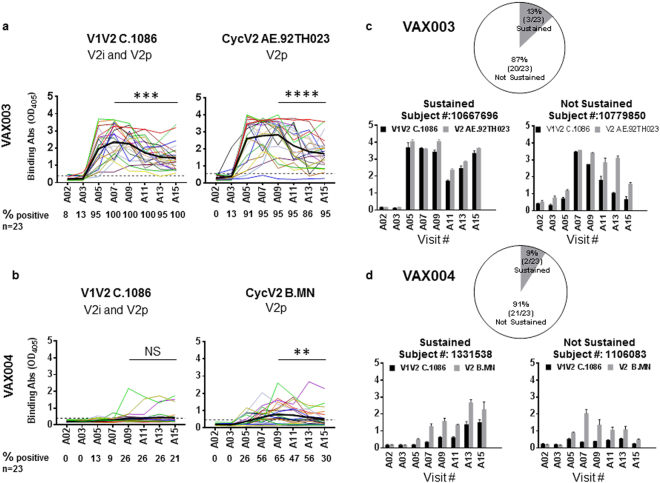
Figure 2.
Anti-V1V2 plasma IgG responses in vaccine recipients over 7 immunization doses. Plasma samples from 23 VAX003 (AIDSVAX® B/E) and 23 VAX004 (AIDSVAX® B/B) trial participants were tested at 1:100 dilution for ELISA IgG reactivity against V1V2-tags (C.1086) expressing V2i and V2p epitopes and cyclic V2 peptides (AE.92TH023 or B.MN) bearing V2p epitopes only. Averages from 2 to 4 replicates tested in 1 or 2 independent experiments are shown. Plasma collection times are described in Fig. 1. (a) Longitudinal analysis compared induction of Ab responses to V1V2-tags (C.1086) and cyclic V2 peptide (AE.92TH023) in VAX003 vaccinees. (b) Similar analysis was performed on VAX004 samples to evaluate the kinetics of Ab responses to V1V2-tags (C.1086) and cyclic V2 peptide (B.MN). OD405, optical density at 405 nm. Percentages of responders (% positive) are shown below each graph. Cut-off values (black dotted lines) were calculated as mean and 3 standard deviations of placebo recipients. Mean antibody levels are highlighted by bold black lines. Comparison of Ab strengths at A15 and peak time points was done by t test (paired, 2-tailed). **P < 0.01, ***P < 0.001; ****P < 0.0001, NS: not significant. (c,d) Few VAX003 and VAX004 vaccinees had sustained Ab responses to V1V2-tags and V2 peptide. The majority of responses to one or both antigens decreased by > 30%. Data of 4 individual vaccine recipients (mean ± standard deviation) with sustained versus not sustained responses are shown.