Fig. 3.
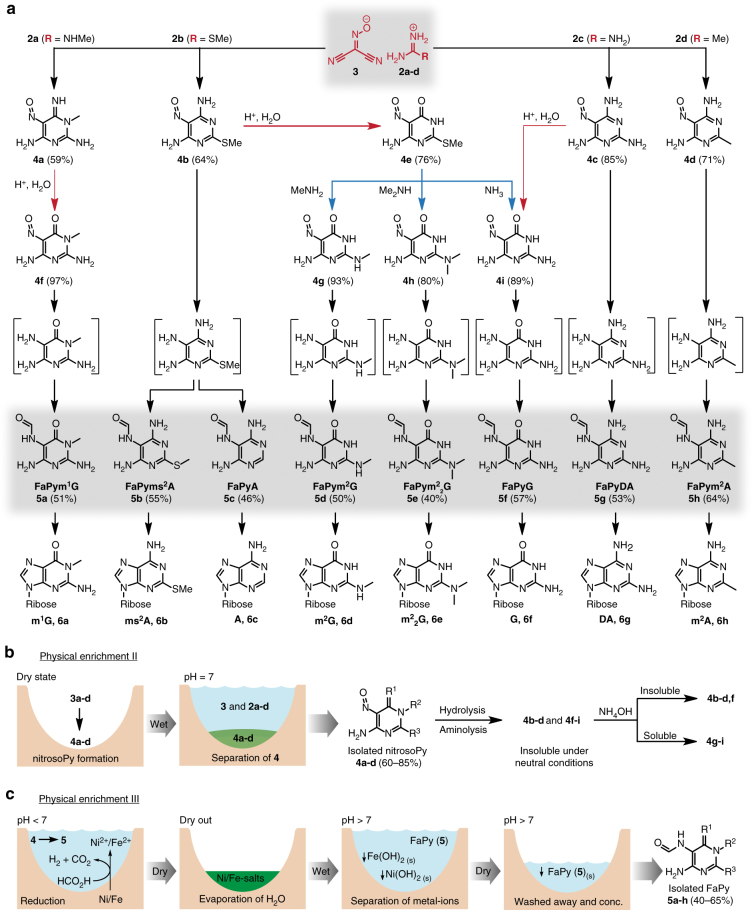
Reaction scheme and physical enrichment of intermediates. a Dry-state reactions of salts containing 2a-d and 3 provide nitroso-pyrimidines 4a-d, which can be further diversified by hydrolysis (red arrows) or aminolysis (blue arrows) to give a set of nitroso-pyrimidines (nitrosoPys) 4a-i. In the presence of elementary Fe and Ni and dilute formic acid, formation of the formamidopyrimidines (FaPys) 5a-h as direct purine base precursors takes place. In square brackets: non-isolated reaction intermediates. b Second physical enrichment of the nitroso-pyrimidines isolated in high purity and yield. c Third physical enrichment of the formed FaPys 5a-h as nucleoside precursors from nitroso-pyrimidines