Abstract
In the present study, we investigated the distribution of CXCL14 immunoreactive endocrine cells and neurons in mouse alimentary tract by immunohistochemistry. CXCL14 immunoreactive endocrine cells were found as closed-type cells in the stomach and open-type cells in the small intestine. The immunostaining of these endocrine cells corresponded with that of the somatostatin-containing endocrine cells. Only a few CXCL14 immunoreactive endocrine cells were seen in the large intestine. CXCL14 immunoreactive fibers were observed in the muscular layer from the stomach to the rectum with most abundance in the rectum. Many CXCL14 immunoreactive fibers were observed in the lamina propria and submucosal layer from the duodenum to the rectum with most abundance in the rectum; these fibers corresponded to the somatostatin-containing nerve fibers. Some CXCL14 immunoreactive neuronal somata that were also immuno-positive for somatostatin, were noted in the submucosal layer of the rectum. However, the remaining parts of the alimentary tract presented with almost negligible immunoreactive somata. The co-localization of CXCL14 and somatostatin suggests that CXCL14 contributes to the function of somatostatin, which include the inhibition of other endocrine and exocrine cells and the enteric nervous systems.
Keywords: CXCL14, chemokine, somatostatin, gut, mouse
I. Introduction
Chemokines are basic proteins (molecular size, 8–12 kDa) that fundamentally possess chemotactic activity for leukocytes and lymphocytes. Based on the position of the cysteines near the amino terminal region, chemokines are divided into four subfamilies, CC, CXC, C, and CX3C [9]. So far, 17 CXC type chemokines have been identified [56]; the CXC subfamily is further divided into ELR+ and ELR− subtypes depending on the presence of “Glu-Leu-Arg” residues at the N-terminals [29].
CXCL14 is a member of the CXC subfamily and belongs to the ELR- subtype. CXCL14 was first isolated from human breast and kidney cells and originally termed as BRAK [19]. The full-length cDNA of CXCL14 encodes 111 amino acids with a putative signal peptide of 34 amino acids [7]. CXCL14 is expressed in epithelial tissues and mesenchyme-derived cells throughout the body [32]. However, it is not expressed in majority of the head and neck squamous cell carcinomas, and other established human cancers cell lines [11, 19]. Several physiological functions of this chemokine such as recruitment and maturation of monocyte-derived macrophages and renewal of Langerhans cells in the skin have been proposed [24, 45]. The other functions of CXCL14 include stimulation of trafficking of activated natural killer cells to the sites of inflammation or malignancy [50], infiltration of macrophages into white adipose tissue under a high-fat diet [34], and inhibition of angiogenesis [47]. Suppression of tumor formation by this chemokine has also been reported [36]. In addition to these functions in the peripheral organs, CXCL14 is thought to possess neuron-associated functions as neuromodulators [3, 46, 55] and regulators of neuronal migration [37].
Although CXCL14 mRNA is present in normal and cancerous tissues of the alimentary tract [28], its protein expression in various cell types within the alimentary tract is not fully elucidated. The gastro-enteric endocrine cells regulate exocrine and endocrine gastrointestinal secretion, motility, blood perfusion and cell renewal under physiological and pathological conditions in tandem with the gastro-enteric nervous system. These endocrine cells, in general, are divided into two groups depending on the extent of the apical cytoplasmic processes in the intestinal lumen. The apical processes in open-type endocrine cells contact the intestinal lumen and receive chemical information from the intestinal contents. On the other hand, the processes in the closed-type endocrine cells do not contact the intestinal lumen; therefore, the cells regulate themselves and/or other endocrine and exocrine cells through autocrine, paracrine, and/or endocrine fashions. To date abundant peptidergic and monoaminergic gastro-enteric endocrine cells have been discovered and are classified into six categories based on their structural and functional similarities [8]. The enteric nervous system controls and modulates various functions of the gut [12]. Neurons located in the submucosal (Meissner’s) and intermuscular (Auerbach’s) nerve plexus control gut functions by releasing a mixture of different inhibitory and/or excitatory neurotransmitters [13]. These neurotransmitters are expressed in distinct classes of enteric neurons with different functions [5]. In the present study, we investigated the presence of CXCL14-like immunoreactivity in mouse alimentary tract. This study demonstrates the co-existence of CXCL14-like immunoreactivity with somatostatin in gastro-enteric endocrine cells and in somatostatinergic neurons of the lamina propria and submucosal layer.
II. Materials and Methods
Animals and fixation
Male mice (C57BL/6NCrSlc; n = 7) were deeply anesthetized with Halothane Fluothane (Takeda Pharmaceutical Co. Ltd., Osaka, Japan). The animals were then perfused with 0.85% NaCl, and subsequently, with 4% formaldehyde and 0.2% picric acid in 0.1 M sodium phosphate buffer (PB, pH 6.9). The alimentary tract from the stomach to the rectum was rapidly dissected and separated into the following parts: stomach, upper intestine including duodenum and jejunum, lower intestine (ileum), cecum, colon, and rectum. Each part was longitudinally opened in order to wash out the luminal contents with saline, and fixed for one or two days at 4°C. Subsequently, the samples were embedded in 10% gelatin, blocked and stored in PB. All procedures were carried out under the authority of Institutional Animal Care and Use Committee of Kanagawa Dental University, employing the guidelines established by the committee.
Immunohistochemistry
Each block was immersed in 20% sucrose, and cut into 20 μm-thick sections with a sliding microtome equipped with a frozen stage. The sections were stocked in 0.1 M PB (pH 7.4) containing 0.9% saline (PBS). Immunostaining was performed according to the methods described previously [55]. Briefly, the sections were washed in PBS overnight, and incubated with rabbit anti-human CXCL14 antibody (No. 500-P237; PeproTech Inc., Rocky Hill, N.J., USA) diluted to 0.5 μg/ml in PBS containing 1% bovine serum albumin (BSA) and 0.3% Triton X-100 (PBS-BSAT), for 24 h at 4°C. The antibody was purified by affinity chromatography employing an immobilized human CXCL14 matrix. After washing in PBS, the sections were incubated with biotinylated goat anti-rabbit IgG (BA-1000; Vector Laboratories, Burlingame, Calif., USA) diluted to 1:100 in PBS-BSAT for 1 hr at room temperature. The sections were then washed again in PBS and incubated with avidin-biotin-horseradish peroxidase complex (PK-6100; Vector Laboratories) diluted to 1:200 in PBS-BSAT for 30 min at room temperature. After a final wash in PBS, the sections were reacted with 0.02% 3,3'-diaminobenzidine tetrahydrochloride (DAB) and 0.005% hydrogen peroxide in 0.05 M Tris-HCl buffer solution (pH 7.4). Thereafter, the sections were counterstained with thionin and coverslipped using Malinol (Muto Pure Chemicals, Tokyo, Japan). Controls for anti-CXCL14 antibody were prepared by omitting the antibody during the first incubation. Preliminary experiments indicated that CXCL14 immunoreactive cells corresponded to somatostain-containing endocrine cells. Hence, a few of the stomach samples were embedded in paraffin, cut into three 4 μm-thick consecutive sections and dewaxed. One section was stained with the antibody pre-absorbed with recombinant human CXCL14 (5 μg/ml, No. 300-50; PeproTech Inc.), the middle section was stained with anti-CXCL14 antibody, and the third section was stained with the antibody pre-absorbed with synthetic somatostatin (50 μg/ml, 4023-v; Peptide Institute, Osaka, Japan) to test the specificity of antibody.
Identification of immunoreactive endocrine cell types and nerve fibers
To determine the types of CXCL14 immunoreactive endocrine cells and enteric neurons, double immunofluorescence staining was performed using rat anti-somatostatin serum (NP-105 SSTrat; Protos Biotech Corporation, New York, N.Y., USA) diluted to 1:500 in PBS-BSAT. Immunoreactivities were visualized by Alexa Fluor 555-labeled donkey anti-rabbit IgG (Abcam, Cambridge, UK) for CXCL14 and Alexa Fluor 488-labeled donkey anti-rat IgG (Abcam) for somatostatin.
Immunoelectron microscopy
Immunoelectron microscopy was performed as described in our previous studies [23, 38, 54]. For the pre-embedding method, portions of the stomach and rectum dissected from similarly perfused mice were used. Small blocks of the fixed samples were cut into 20 μm-thick sections and processed; CXCL14 immunoreactivity was detected by DAB reaction. The sections were then fixed in 1% OsO4 for 1 hr at room temperature, dehydrated and embedded in Quetol 812 (Nissin EM, Tokyo, Japan). Subsequently, ultrathin sections were obtained and mounted on nickel grids. After staining with uranyl acetate and lead citrate for 1 min each, the sections were examined under an electron microscope (JEM 1220: JEOL, Tokyo, Japan).
III. Results
Stomach
In the esophageal region of the mouse stomach no CXCL14 immunoreactive cells was observed in the stratified squamous cell layers; however, a few CXCL14 immunoreactive fibers were seen in the myenteric nervous plexus. On the other hand, in the columnar epithelial regions, CXCL14 immunoreactive cells were scattered in the mouse stomach. They were especially abundant in the fundus, and whereas some cells were found in the neck, none was noted in the surface mucous cell layer. These immunoreactive cells often surrounded other types of cells in the epithelial cell layer of the stomach and appeared to be the closed-type of gastro-enteric endocrine cells (Fig. 1A, B). The shapes of the immunoreactive cells varied from triangular to bipolar (width, 5.8–8.2 μm; length, 8.2–23.5 μm). Many immunoreactive fibers were seen running along with long axes of the smooth muscle in the muscular layers (Fig. 1C). Nevertheless, no immunoreactive fibers were noted in the lamina propria and submucosa.
Fig. 1.
CXCL14 immunoreactive endocrine cells in mucosal epithelial cell layer (A, B) and fibers in muscular layer (C) in the columnar epithelial regions of the stomach. Sagittal (A) and transverse (B) sections obtained from the villi. Note that these cells appear to be the closed-type of endocrine cells. Arrows in A and B indicate CXCL14 immunoreactive cells, and arrows in C indicate CXCL14 immunoreactive nerve fibers.
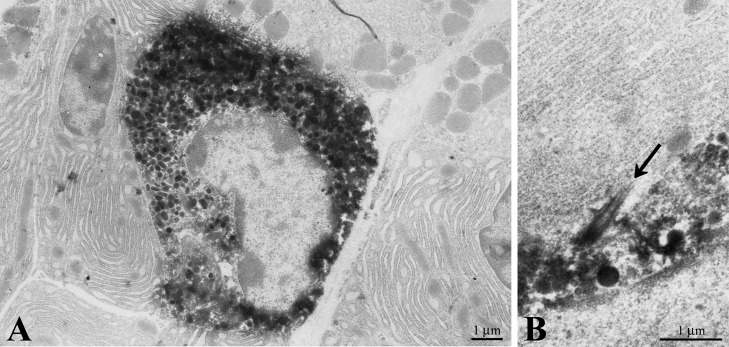
Electron microscopic studies indicated that the CXCL14 immunoreactive cells contained spindle- to horseshoe-shaped nuclei and secretory granules (approximately 160–330 nm in diameter; Fig. 2A). These cells sometimes harbored the cilium that penetrated between the CXCL14 immunoreactive cells and other epithelial cells (Fig. 2B).
Fig. 2.
Electron micrographs showing a CXCL14 immunoreactive cell in the columnar epithelial cell layer (A) and an immunoreactive cell bearing a cilium (B) in the stomach. Arrow in B indicates a cilium protruding from the CXCL14 immunoreactive endocrine cell.
Small intestine
In the duodenum, characterized by the presence of Brunner’s glands beneath the lamina muscularis propria, a few immunoreactive cells, considered to be the open-typed gastro-enteric endocrine cells (data not shown), were found scattered between the duodenal epithelial cells. Unlike in the stomach, some immunoreactive fibers were observed in the lamina propria and submucosa (data not shown), but only a few in the muscular layer.
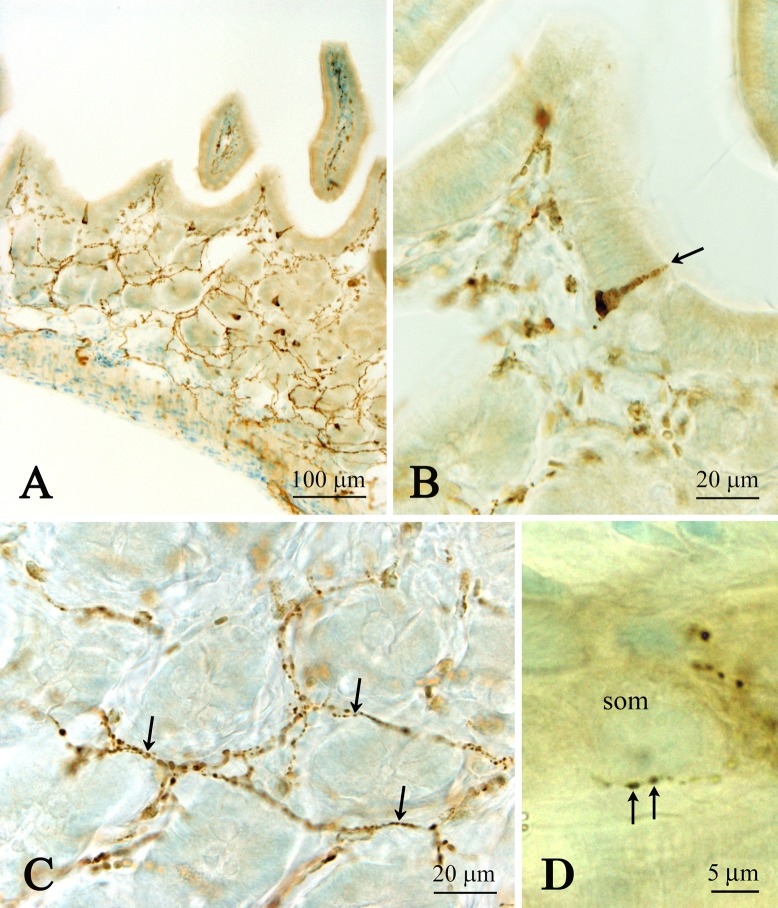
A number of immunoreactive open-typed endocrine cells (the apical processes seemed to reach the lumen) were seen in the epithelial cell layer of the jejunum (Fig. 3A, B). Immunoreactive fibers were abundant in the lamina propria and submucosa of the jejunum (Fig. 3A, C) when compared to those of the duodenum. These fibers were found to innervate the tip of villi and often surrounded the neuronal somata in the Meissner’s nerve plexus (Fig. 3D). A few fibers were also localized in the muscular layer, including the myenteric nervous plexus. In the ileum, only a few immunoreactive cells were observed with some immunoreactive fibers in the lamina propria and submucosa, and only a few in the muscular layer (data not shown).
Fig. 3.
Low (A) and high (B, C, D) magnification images showing CXCL14 immunoreactive endocrine cells (A, B) and nerve fibers (A, C, D) in the jejunum. Note that the CXCL14 immunoreactive cells seem to be the open-typed (B). CXCL14 immunoreactive fibers surrounding the villi (C). (D) Close apposition of CXCL14 immunoreactive fibers on neuronal somata (som) located in the submucosal nerve plexus. Arrow in B indicates CXCL14 immunoreactive endocrine cell. Arrows in C and D indicate CXCL14 immunoreactive nerve fibers.
Large intestine
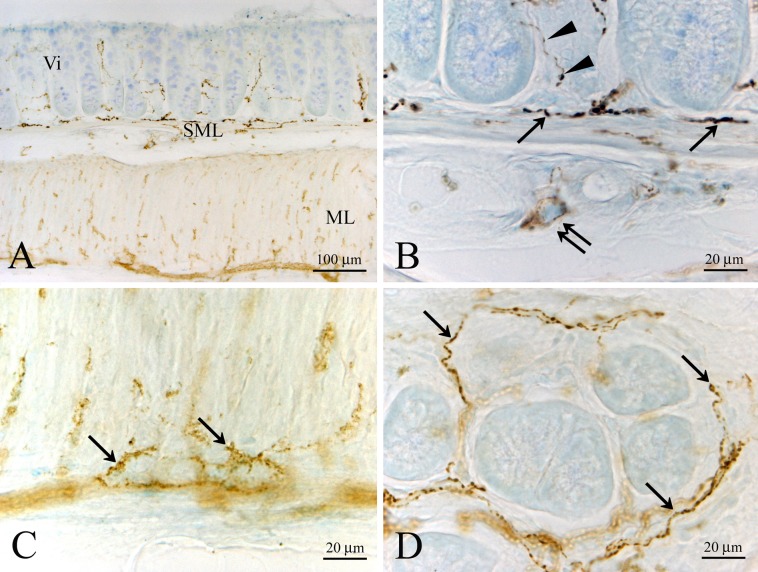
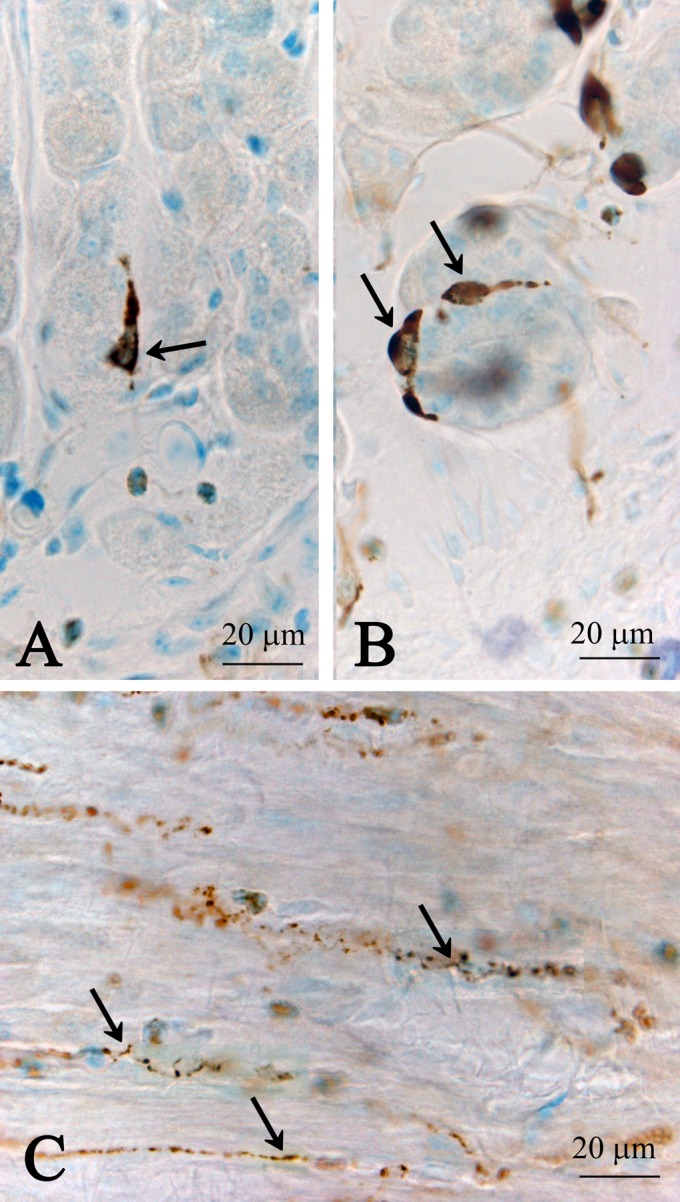
Only a few immunoreactive cells were observed in the epithelial cell layer of the cecum. Some immunoreactive fibers were seen in the lamina propria, submucosa and muscular layer (data not shown). In the colon, no immunoreactive cells were seen in the epithelial layer. However, immunoreactive fibers were abundant in the lamina propria, submucosa and muscular layer (data not shown). In the rectum, we found a few immunoreactive cells that seemed to be of the closed-type of endocrine cells in the epithelial cell layer (data not shown). On the other hand, abundant immunoreactive fibers were seen in the lamina propria, submucosa and muscular layer (Fig. 4A–D). In the rectum, some immunoreactive somata were noted in the submucosa (Fig. 5A, B). The short and long axes of these somata were approximately 4.0 μm and 12.0 μm, respectively. Electron microscopic studies indicated that CXCL14 immunoreactive fibers in the submucosa contained clear synaptic vesicle-like structures (approximately 45–60 nm in diameter; Fig. 6), which is one of the characteristic features of the nerve fibers.
Fig. 4.
Low (A) and high (B, C, D) magnification images showing CXCL14 immunoreactive fibers in the lamina propria, submucosal layer (SML) and muscular layer (ML) of the rectum. CXCL14 immunoreactive fibers are concentrated at the bottom of the villi (denoted as Vi in A and arrows in B). (B) An immunoreactive neuronal soma (double arrows) in the submucosal nerve plexus. Arrowheads in B indicate CXCL14 immunoreactive fibers in the lamina propria. (C) CXCL14 immunoreactive fibers (arrows) in the muscular layer. (D) CXCL14 immunoreactive fibers (arrows) surrounding the villi.
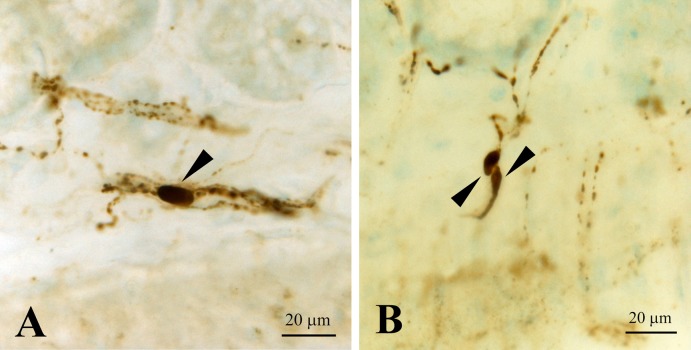
Fig. 5.
CXCL14 immunoreactive neuronal somata (arrowheads) in the submucosal layer of the rectum. These CXCL14 immunoreactive fibers are sometimes parallel to the submucosal layer (A), and sometimes run across the layer (B).
Fig. 6.
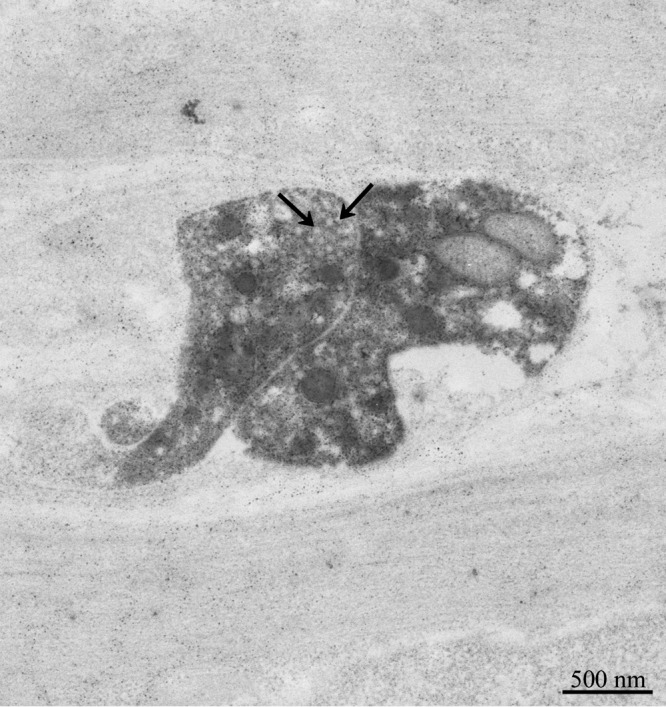
Electron micrograph showing CXCL14 immunoreactive fibers in the rectum submucosa. Arrows indicate clear synaptic vesicle-like structures.
Double labeling
A double labeling study indicated that all CXCL14 immunoreactive cells were immuno-positive for somatostatin in the stomach (Fig. 7A–D) and jejunum (Fig. 7E, F). CXCL14 immunoreactive fibers in the lamina propria of the small and large intestines were immuno-positve for somatostatin (Fig. 8A, B). CXCL14 immunoreactive neuronal somata in the submucosa of the rectum were also immuno-positive for somatostatin (Fig. 8C, D). However, the CXCL14 immunoreactive fibers in the muscular layer from the stomach to the rectum were immuno-negative for somatostatin (Fig. 8E, F).
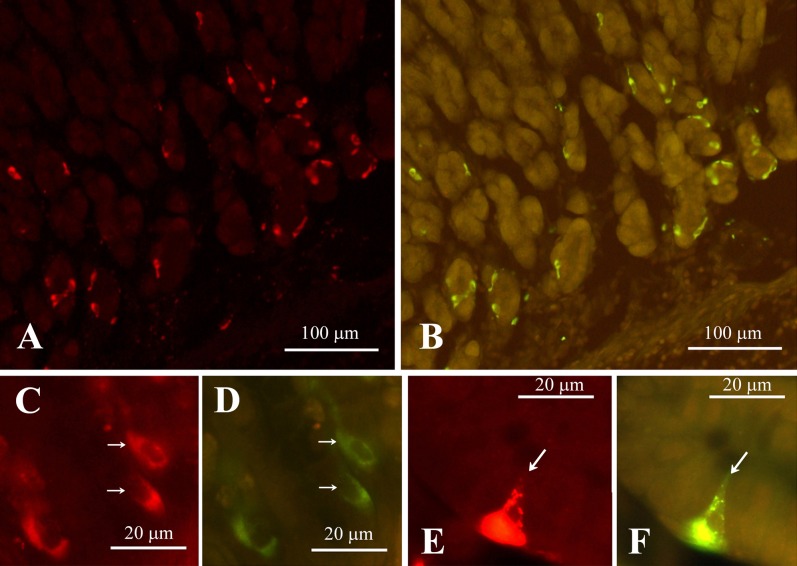
Fig. 7.
Double fluorescent immunostaing of CXCL14 (A, C, E) and somatostatin (B, D, F) in the stomach (A–D) and jejunum (E, F). Note that the immunostainig of all the CXCL14 cells (arrows in C and E) corresponded with that of the somatostatin cells (arrows in D and F). (A) and (B), (C) and (D), and (E) and (F) are identical sections.
Fig. 8.
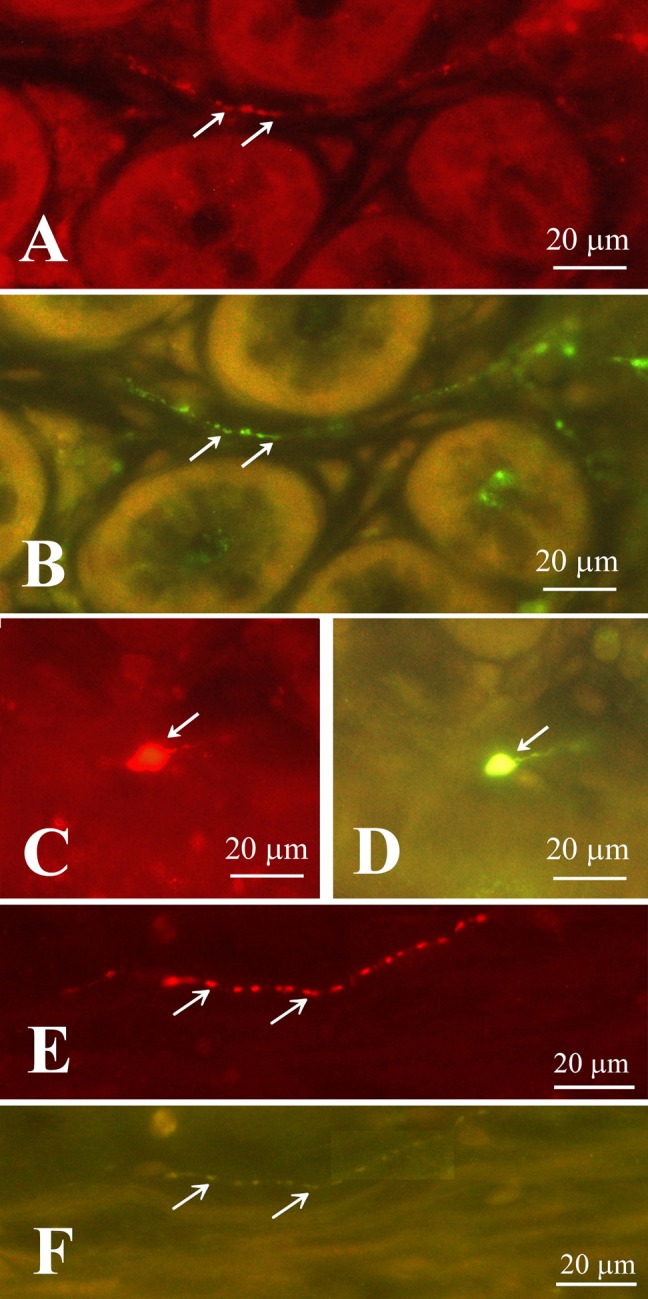
Double fluorescent immunostaining of CXCL14 (A, C, E) and somatostatin (B, D, F) in the rectum. Arrows in A and B indicate co-labeled fibers; arrow in C and D indicates a co-labeled soma. However, arrows in E and F indicate no co-localization of CXCL14-like immunoreactivity in the somatostatinergic nerve fibers in the muscular layer. (A) and (B), (C) and (D), and (E) and (F) are identical sections.
Table 1 shows the relative densities and co-localization states with somatostatin of CXCL14 immunoreactive endocrine cells and nerve fibers.
Table 1. .
Relative density of CXCL14 immunoreactive endocrine cells and fibers
| stomach | duodenum | jejunum | ileum | cecum | colon | rectum | |
|---|---|---|---|---|---|---|---|
| Endocrine cells (co-localized with SST) | +++ | + | ++ | + | + | − | + |
| Fibers in lamina propria and submucosa (co-localized with SST) | − | ++ | +++ | ++ | ++ | +++ | +++ |
| Fibers in muscular layer (not co-localized with SST) | ++ | + | + | + | ++ | +++ | +++ |
Abbreviation: SST, somatostatin. Frequency of staining profiles: +++, abundant; ++, some; +, a few; −, none.
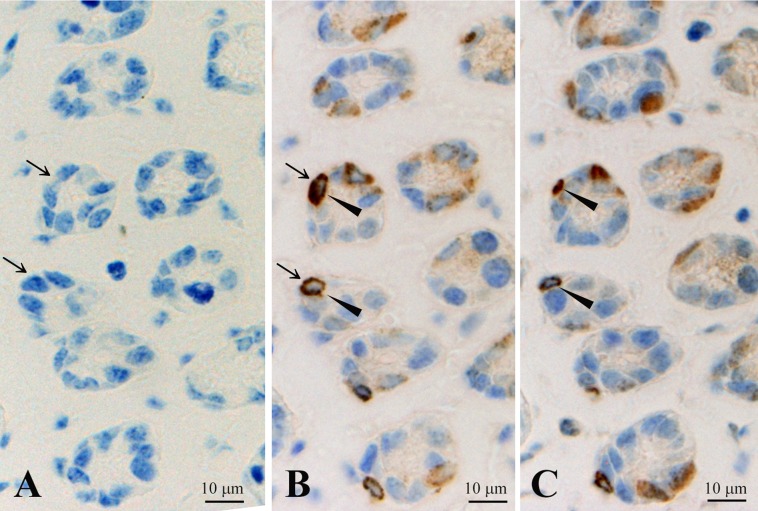
Pre-absorption tests
Pre-absorption of the antibody with CXCL14 resulted in the disappearance of immunoreactive cells in the columnar epithelial cells of the stomach (Fig. 9A, B); however, pre-absorption of the antibody with somatostatin had no effects on CXCL14 staining profiles (Fig. 9B, C).
Fig. 9.
Three consecutive sections of the stomach indicating effects of pre-absorption of anti-CXCL14 antibody used in this study with recombinant CXCL14 (A) and somatostatin (C). (B) Positive control. Arrows in A and B indicate the cells in which CXCL14-like immunoreactivity disappears following pre-absorption with recombinat CXCL14; arrowheads in B and C indicate the cells in which CXCL14-like immunoreactivity was not affected by pre-abosorption with somatostatin.
IV. Discussion
Gastro-entero-pancreatic (GEP) endocrine cells contribute to the regulation of gastrointestinal systems [8]. We have previously demonstrated that CXCL14-like immunoreactivity is localized in somatostatin immunoreactive cells in mouse pancreatic islets [51]. In the present study, we further confirmed the co-localization of these two peptides. In explanation, CXCL14 immunoreactive cells in the stomach were closed-type of somatostatin-containing cells and those in the small intestine were open-type of somatostatin-containing cells. In general, the open-type of GEP endocrine cells have apical cytoplasmic processes that make contact with and receive chemical information from the gastrointestinal contents. In contrast, the closed-type of GEP endocrine cells seem to sense local chemical and mechanical information [8]. The presence of open-type and closed-type of CXCL14/somatostatin cells is accord with the previous studies demonstrating that somatostatin immunoreactive cells contained both open- and closed-type GEP endocrine cells in other animals, including rat, dog, cat, and sheep [1, 6, 22, 25, 27]. Somatostatin suppresses gastrin release from G cells located in the stomach [17]. Although the functions of CXCL14 located on GEP endocrine cells are not known so far, co-localization suggests that CXCL14 contributes to the endocrine functions of somatostatin, similar to the pancreatic somatostatin cells [51].
A principal function of somatostatin is the suppression of all activities in the gastrointestinal tract [26], such as exocrine activities [30] and the inhibition of gastrointestinal hormone secretion including gastrin release as noted above [26, 30]. Furthermore, somatostatin inhibits gastrointestinal motility and decreases visceral blood flow, cell growth and cell renewal in concert with somatostatin-containing enteric neuronal system. On the other hand, somatostatin release is induced by various factors, which include not only fat and proteins [39], but also various hormones such as CCK, VIP and gastrin [10, 18]. Interestingly, in accordance with previous studies [16, 43], CXCL14/somatostatin immunoreactive endocrine cells in the stomach were found to contain cilia that protruded into the space between the immunoreactive and mucous epithelial cells. Cilia are fundamentally sensory in nature and mainly detect their environment [44, 48]. Somatostatin cells in the stomach are regarded as chemical sensory cells not only for meal but also hormones. Evidently, the cilia themselves contain hormone receptors such as somatostatin receptors [2, 15, 20]. Therefore, the cilia in the CXCL14/somatostatin cells may be responsible for sensation of local hormonal and chemical conditions. In addition, stomach CXCL14/somatostatin immunoreactive cells belong to the closed-type of endocrine cells, which suggests its role as a mechanoreceptor that detects the extension of the mucous layer and contributes to bowel reflex. Supportingly, it is reported that somatostatin cells react to the extension of stomach [43].
The enteric nervous system consists of two main plexuses, submucosal plexus and myenteric plexus, and regulates gastrointestinal absorption, secretion and motility [12]. It contains various types of neurons, which based on morphological, physiological and neurochemical characteristics, are classified into 18 types in the guinea pig small intestine [5]. CXCL14 immunoreactive fibers in the lamina propria and submucosal layer corresponded to somatostatin-containing fibers. CXCL14 immunoreactive neuronal somata were mainly located in the submucosal nerve plexus. Submucosal somatostatin-containing neurons belong to secretomotor neurons that project into the mucosa and mucosal glands [5, 49]. On the other hand, myenteric somatostatinergic neurons are inhibitory interneurons with descending (anally) axons [5, 40]. Based on the absence of co-localization of CXCL14 with somatostatin in the muscular layer, CXCL14 may associate more likely with the secretomotor functions of somatostatin, rather than function as an inhibitory interneuron. The nature of CXCL14 as a neuromodulator and/or neurotransmitter is not fully elucidated at present. Nevertheless, the existence of CXCL14 in neurons has been demonstrated in hypothalamic neurons [55] and GABAergic neurons [3, 46]. Furthermore, CXCL14 modulates the expression and maintenance of tyrosin hydroxylase (dopamine synthesis enzyme) levels in mesencephalic dopaminergic neurons [52] and inhibits tonic and phasic effects of synaptically released GABA [3]. Other chemokines also perform not only as chemotactants but also as neuromodulators. For example, CXCL12 stimulates the release of glutamate from astrocytes and affects the synaptic activity independently of its receptors on the neurons [4]. CXCL8 reduces the calcium current in cholinergic neurons [41], and CXCL1 and 8 modulate calcium transients, enhance synaptic activity and suppress the induction of long-term depression in cerebellar Purkinje cells through CXCR2 (a chemokine receptor) [14]. Furthermore, several CC and CXC chemokines are able to reduce calcium oscillation in hippocampal neurons [31]. All these lines suggest that CXCL14 may also serve as a neuromodulator in addition to its chemotactic functions.
To date, the purpose of the coexistence of CXCL14 with somatostatin in GEP endocrine cells and part of the gastrointestinal nervous plexuses remains unclear; moreover, the CXCL14 receptor has not yet been identified. In contrast, somatostatin receptors are well characterized and classified into five subtypes. These somatostatin receptors are often targeted for the therapeutic treatment of various neuroendocrine tumors [42]. Although underling mechanisms of these two peptides-associating functions are not elucidated yet, it is worthy to note that clinically, both CXCL14 and somatostatin have antitumor activities [21, 33, 35, 53]. Further studies on the genetic and protein expression profiles of CXCL14 in various neuroendocrine tumors may clarify the association of these two peptides and their antitumor mechanisms.
V. Conflict of Interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
VI. Acknowledgments
A part of this work was supported by JSPA KAKENHI (Grant number 15K20459 and 15H05058) and the authors thank Miss Mari Sato for her technical assistance.
VII. References
- 1.Alumets J., Ekelund M., El Munshid H. A., Hӓkanson R., Lorén I. and Sundler F. (1979) Topography of somatostatin cells in the stomach of the rat: possible functional significance. Cell Tissue Res. 202; 177–188. [DOI] [PubMed] [Google Scholar]
- 2.Baly C., Aioun J., Badonnel K., Lacroix M. C., Durieux D., Schlegel C., Salesse R. and Caillol M. (2007) Leptin and its receptors are present in the rat olfactory mucosa and modulated by the nutritional status. Brain Res. 1129; 130–141. [DOI] [PubMed] [Google Scholar]
- 3.Banisadr G., Bhattacharyya B. J., Belmadani A., Izen S. C., Ren D., Tran P. B. and Miller R. J. (2011) The chemokine BRAK/CXCL14 regulates synaptic transmission in the adult mouse dentate gyrus stem cell niche. J. Neurochem. 119; 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezzi P., Domercq M., Brambilla L., Galli R., Schols D., De Clercq E., Vescovi A., Bagetta G., Kollias G., Meldolesi J. and Volterra A. (2001) CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 4; 702–710. [DOI] [PubMed] [Google Scholar]
- 5.Brookes S. J. (2001) Classes of enteric nerve cells in the guinea-pig small intestine. Anat. Rec. 262; 58–70. [DOI] [PubMed] [Google Scholar]
- 6.Calingasan N. Y., Kitamura N., Yamada J., Oomori Y. and Yamashita T. (1984) Immunocytochemical study of the gastroenteropancreatic endocrine cells of the sheep. Acta Anat. (Basel) 118; 171–180. [DOI] [PubMed] [Google Scholar]
- 7.Cao X., Zhang W., Wan T., He L., Chen T., Yuan Z., Ma S., Yu Y. and Chen G. (2000) Molecular cloning and characterization of a novel CXC chemokine macrophage inflammatory protein-2γ chemoattractant for human neutrophils and dendritic cells. J. Immunol. 165; 2588–2595. [DOI] [PubMed] [Google Scholar]
- 8.Ceranowicz P., Warzecha Z. and Dembinski A. (2015) Peptidyl hormones of endocrine cells origin in the gut—their discovery and physiological relevance. J. Physiol. Pharmacol. 66; 11–27. [PubMed] [Google Scholar]
- 9.da Silva J. M., Soave D. F., Moreira Dos Santos T. P., Batista A. C., Russo R. C., Teixeira M. M. and da Silva T. A. (2016) Significance of chemokine and chemokine receptors in head and neck squamous cell carcinoma: A critical review. Oral Oncol. 56; 8–16. [DOI] [PubMed] [Google Scholar]
- 10.Eissele R., Koop H. and Arnold R. (1990) Effect of glucagon-like peptide-1 on gastric somatostatin and gastrin secretion in the rat. Scand. J. Gastroenterol. 25; 449–454. [DOI] [PubMed] [Google Scholar]
- 11.Frederick M. J., Henderson Y., Xu X., Deavers M. T., Sahin A. A., Wu H., Lewis D. E., El-Naggar A. K. and Clayman G. L. (2000) In vivo expression of the novel CXC chemokine BRAK in normal and cancerous human tissue. Am. J. Pathol. 156; 1937–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furness J. B., Jones C., Nurgali K. and Clerc N. (2004) Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 72; 143–164. [DOI] [PubMed] [Google Scholar]
- 13.Furness J. B., Young H. M., Pompolo S., Bornstein J. C., Kunze W. A. and McConalogue K. (1995) Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology 108; 554–563. [DOI] [PubMed] [Google Scholar]
- 14.Giovannelli A., Limatola C., Ragozzino D., Mileo A. M., Ruggieri A., Ciotti M. T., Mercanti D., Santoni A. and Eusebi F. (1998) CXC chemokines interleukin-8 (IL-8) and growth-related gene product α (GROα) modulate Purkinje neuron activity in mouse cerebellum. J. Neuroimmunol. 92; 122–132. [DOI] [PubMed] [Google Scholar]
- 15.Händel M., Schulz S., Stanarius A., Schreff M., Erdtmann-Vourliotis M., Schmidt H., Wolf G. and Höllt V. (1999) Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience 89; 909–926. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto Y., Ushiki T., Uchida T., Yamada J. and Iwanaga T. (1999) Scanning electron microscopic observation of apical sites of open-type paraneurons in the stomach, intestine and urethra. Arch. Histol. Cytol. 62; 181–189. [DOI] [PubMed] [Google Scholar]
- 17.Hersey S. J. and Sachs G. (1995) Gastric acid secretion. Physiol. Rev. 75; 155–189. [DOI] [PubMed] [Google Scholar]
- 18.Holzer P. (1998) Neural emergency system in the stomach. Gastroenterology 114; 823–839. [DOI] [PubMed] [Google Scholar]
- 19.Hromas R., Broxmeyer H. E., Kim C., Nakshatri H., Christopherson K. II, Azam M. and Hou Y. H. (1999) Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus malignant cells. Biochem. Biophys. Res. Commun. 255; 703–706. [DOI] [PubMed] [Google Scholar]
- 20.Iwanaga T., Miki T. and Takahshi-Iwanaga H. (2011) Restricted expression of somatostatin receptor 3 to primary cilia in the pancreatic islets and adenohypophysis of mice. Biomed. Res. 32; 73–81. [DOI] [PubMed] [Google Scholar]
- 21.Keskin O. and Yalcin S. (2013) A review of the use of somatostatin analogs in oncology. Onco Targets Ther. 6; 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura N., Yamada J., Yamashita T. and Yanaihara N. (1982) Endocrine cells in the gastrointestinal tract of the cat. Biomed. Res. 3; 612–622. [DOI] [PubMed] [Google Scholar]
- 23.Kojo A., Yamada K. and Yamamoto T. (2016) Glucose transporter 5 (GLUT5)-like immunoreactivity is localized in subsets of neurons and glia in the rat brain. J. Chem. Neuroanat. 74; 55–70. [DOI] [PubMed] [Google Scholar]
- 24.Kurth I., Willimann K., Schaerli P., Hunziker T., Clark-Lewis I. and Moser B. (2001) Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J. Exp. Med. 194; 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusumoto Y., Iwanaga T., Ito S. and Fujita T. (1979) Juxtaposition of somatostatin cell and parietal cell in the dog stomach. Arch. Histol. Jpn. 42; 459–465. [DOI] [PubMed] [Google Scholar]
- 26.Lamers C. B. (1987) Clinical and pathophysiological aspects of somatostatin and the gastrointestinal tract. Acta Endocrinol. Suppl. (Copenh) 286; 19–25. [DOI] [PubMed] [Google Scholar]
- 27.Larsson L.-I., Goltermann N., de Magistris L., Rehfeld J. F. and Schwartz T. W. (1979) Somatostatin cell processes as pathways for paracrine secretion. Science 205; 1393–1395. [DOI] [PubMed] [Google Scholar]
- 28.Leja J., Essaghir A., Essand M., Wester K., Oberg K., Tötterman T. H., Lloyd R., Vasmatzis G., Demoulin J. B. and Giandomenico V. (2009) Novel markers for enterochromaffin cells and gastrointestinal neuroendorine carcinomas. Mod. Pathol. 22; 261–272. [DOI] [PubMed] [Google Scholar]
- 29.Liu X., Dai L. and Zhou R. (2015) Association between preeclampsia and the CXC chemokine family (Review). Exp. Ther. Med. 9; 1572–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low M. J. (2004) Clinical endocrinology and metabolism. The somatostatin neuroendocrine system: physiology and clinical relevance in gastrointestinal and pancreatic disorders. Best Pract. Res. Clin. Endocrinol. Metab. 18; 607–622. [DOI] [PubMed] [Google Scholar]
- 31.Meucci O., Fatatis A., Simen A. A., Bushell T. J., Gray P. W. and Miller R. J. (1998) Chemokines regulate hippocampal neuronal signaling and gp 120 neurotoxicity. Proc. Natl. Acad. Sci. U S A 95; 14500–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meuter S. and Moser B. (2008) Constitutive expression of CXCL14 in healthy human and murine epithelial tissues. Cytokine 44; 248–255. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto C., Maehata Y., Ozawa S., Ikoma T., Kubota E., Izukuri K., Kato Y., Hata R. and Lee M. C. (2012) Fasudil suppresses fibrosarcoma growth by stimulating secretion of the chemokine CXCL14/BRAK. J. Pharmacol. Sci. 120; 241–249. [DOI] [PubMed] [Google Scholar]
- 34.Nara N., Nakayama Y., Okamoto S., Tamura H., Kiyono M., Muraoka M., Tanaka K., Taya C., Shitara H., Ishii R., Yonekawa H., Minokoshi Y. and Hara T. (2007) Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J. Biol. Chem. 282; 30794–30803. [DOI] [PubMed] [Google Scholar]
- 35.Oberg K. E., Reubi J. C., Kwekkeboom D. J. and Krenning E. P. (2010) Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 139; 742–753. [DOI] [PubMed] [Google Scholar]
- 36.Ozawa S., Kato Y., Ito S., Komori R., Shiiki N., Tsukinoki K., Ozono S., Maehata Y., Taguci T., Imagawa-Ishiguro Y., Tsukuda M., Kubota E. and Hata R. (2009) Restoration of BRAK/CXCL14 gene expression by gefitinib is associated with antitumor efficacy of the drug in head and neck squamous cell carcinoma. Cancer Sci. 100; 2202–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park C. R., Kim D.-K., Cho E. B., You D.-J., do Rego J. L., Vaudry D., Sun W., Kim H., Seong J. Y. and Hwang J.-I. (2012) Spatiotemporal expression and functional implication of CXCL14 in the developing mice cerebellum. Mol. Cells 34; 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park H., Yamada K., Kojo A., Sato S., Onozuka M. and Yamamoto T. (2009) Drebrin (developmentally regulated brain protein) is associated with axo-somatic synapses and neuronal gap junctions in rat mesencephalic trigeminal nucleus. Neurosci. Lett. 461; 95–99. [DOI] [PubMed] [Google Scholar]
- 39.Penman E., Wass J. A., Medbak S., Morgan L., Lewis J. M., Besser G. M. and Rees L. H. (1981) Response of circulating immunoreative somatostatin to nutritional stimuli in normal subjects. Gastroenterology 81; 692–699. [PubMed] [Google Scholar]
- 40.Portbury A. L., Pompolo S., Furness J. B., Stebbing M. J., Kunze W. A., Bornstein J. C. and Hughes S. (1995) Cholinergic, somatostatin-immunoreactive interneurons in the guinea pig intestine: morphology, ultrastucture, connections and projections. J. Anat. 187; 303–321. [PMC free article] [PubMed] [Google Scholar]
- 41.Puma C., Danik M., Quirion R., Ramon F. and Williams S. (2001) The chemokine interleukin-8 acutely reduces Ca2+ currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J. Neurochem. 78; 960–971. [DOI] [PubMed] [Google Scholar]
- 42.Reubi J. C. (2004) Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology 80 Suppl. 1; 51–56. [DOI] [PubMed] [Google Scholar]
- 43.Saqui-Salces M., Dowdle W. E., Reiter J. F. and Merchant J. L. (2012) A high-fat diet regulates gastrin and acid secretion through primary cilia. FASEB J. 26; 3127–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satir P., Pedersen L. B. and Christensen S. T. (2010) The primary cilium as a glance. J. Cell Sci. 123; 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaerli P., Willimann K., Ebert L. M., Walz A. and Moser B. (2005) Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation. Immunity 23; 331–342. [DOI] [PubMed] [Google Scholar]
- 46.Schmid, C. D., Melchior B., Masek K., Puntambekar S. S., Danielson P. E., Lo D. D., Sutcliffe J. G. and Carson M. J. (2009) Differential gene expression in LPS/IFNγ activated microglia and macrophages: in vitro vesus in vivo. J. Neurochem. 109 Suppl. 1; 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shellenberger T. D., Wang M., Gujrati M., Jayakumar A., Strieter R. M., Burdick M. D., Ioannides C. G., Efferson C. L., El-Naggar A. K., Roberts D., Clayman G. L. and Frederick M. J. (2004) BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 64; 8262–8270. [DOI] [PubMed] [Google Scholar]
- 48.Singla V. and Reiter J. F. (2006) The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313; 629–633. [DOI] [PubMed] [Google Scholar]
- 49.Song Z. M., Brookes S. J., Steele P. A. and Costa M. (1992) Projections and pathways of submucous neurons to the mucosa of the guinea-pig small intestine. Cell Tissue Res. 269; 87–98. [DOI] [PubMed] [Google Scholar]
- 50.Starnes T., Rasila K. K., Robertson M. J., Brahmi Z., Dahl R., Christopherson K. and Hromas R. (2006) The chemokine CXCL14 (BRAK) stimulates activated NK cell migration: implications for the downregulation of CXCL14 in malignancy. Exp. Hematol. 34; 1101–1105. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki H. and Yamamoto T. (2015) CXCL14-like immunoreactivity exists in somatostatin-containing cells of mouse pancreas. Acta Histochem. Cytochem. 48; 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vadasz C., Smiley J. F., Figarsky K., Saito M., Toth R., Gyetvai B. M., Oros M., Kovacs K. K., Mohan P. and Wang R. (2007) Mesencephalic dopamine neuron number and tyrosine hydroxylase content: genetic control and candidate genes. Neuroscience 149; 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W., Huang P., Zhang L., Wei J., Xie Q., Sun Q., Zhou X., Xie H., Zhou L. and Zheng S. (2013) Antitumor efficacy of C-X-C motif chemokine ligand 14 in hepatocellular carcinoma in vitro and in vivo. Cancer Sci. 104; 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada K., Park H., Sato S., Onozuka M., Kubo K. and Yamamoto T. (2008) Dynorphin-A immunoreactive terminals on the neuronal somata of rat mesencephalic trigeminal nucleus. Neurosci. Lett. 438; 150–154. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto T., Yamashita A., Yamada K. and Hata R. (2011) Immunohistochemical localization of chemokine CXCL14 in rat hypothalamic neurons. Neurosci. Lett. 487; 335–340. [DOI] [PubMed] [Google Scholar]
- 56.Zlotnik A., Yoshie O. and Nomiyama H. (2006) The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 7; 243. [DOI] [PMC free article] [PubMed] [Google Scholar]