Figure 3.
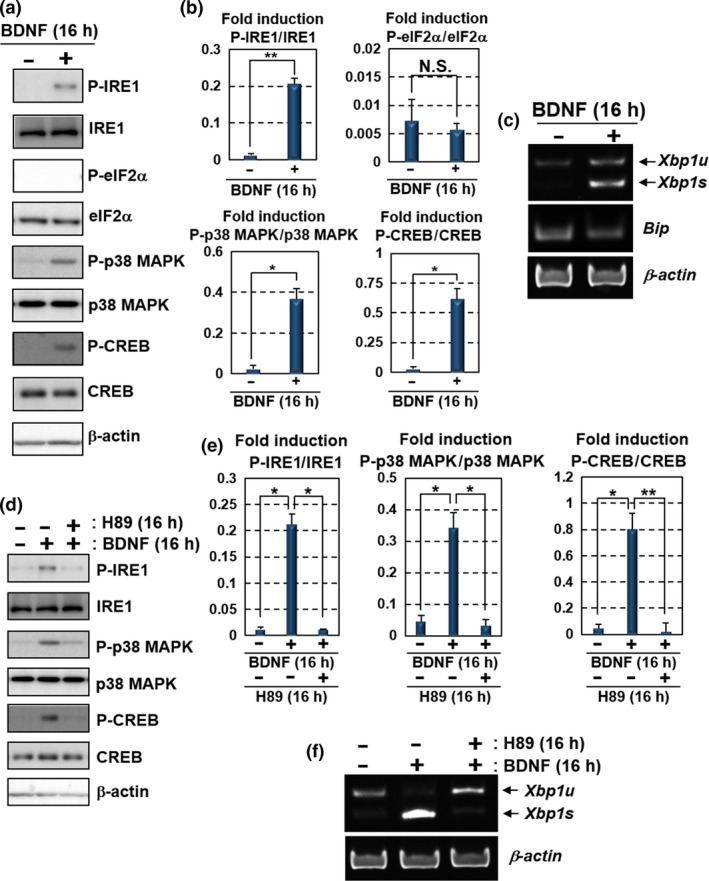
BDNF up‐regulates the IRE1‐XBP1 pathway via PKA activation. Primary cultured mouse hippocampal neurons maintained for 10 days were used for the analyses. (a) Western blot analysis of P‐IRE1, IRE1, P‐eIF2α, eIF2α, phosphorylated p38 MAPK (P‐p38 MAPK), p38 MAPK, phosphorylated CREB (P‐CREB) and CREB. Cells were treated with 100 ng/mL BDNF for 16 h. IRE1, p38 MAPK and CREB, but not eIF2α, were phosphorylated after the BDNF treatment. (b) Quantification of relative protein levels of P‐IRE1, P‐eIF2α, P‐p38 MAPK and P‐CREB in (a) (mean ± SD, N = 3; number of extracted samples prepared from independent cultures; *p < 0.05, **p < 0.01). (c) RT‐PCR analysis of Xbp1 and Bip mRNA. Xbp1s was increased by the treatment with 100 ng/mL BDNF for 16 h, but not Bip. (d) Western blot analysis of P‐IRE1, IRE1, P‐p38 MAPK, p38 MAPK, P‐CREB and CREB. The cells were treated with 100 ng/mL BDNF and 30 μM H89 (PKA inhibitor) for 16 h. The phosphorylation levels of IRE1, p38 MAPK and CREB induced by the treatment with BDNF were attenuated by treatment with H89. (e) Quantification of relative protein levels of P‐IRE1, P‐p38 MAPK and P‐CREB in (d) (mean ± SD, N = 3; number of extracted samples prepared from independent cultures; *p < 0.05, **p < 0.01). (f) RT‐PCR analysis of Xbp1 mRNA. The acceleration of Xbp1 splicing by the treatment with 100 ng/mL BDNF was inhibited by the treatment with 30 μM H89 for 16 h.
