Abstract
Aim
To evaluate the non‐inferiority of the adjunct of a xenogeneic collagen matrix (CMX) or connective tissue graft (CTG) to coronally advanced flaps (CAF) for coverage of multiple adjacent recessions and compare superiority in patient‐reported outcomes (PROM).
Material and methods
One hundred and eighty‐seven subjects (92 CMX) with 485 recessions in 14 centres were randomized and followed up for 6 months. Patients filled daily diaries for 15 days to monitor patient‐reported experience. The primary outcome was changed in position of the gingival margin. Multilevel analysis used centre, subject and tooth as levels and baseline parameters as covariates.
Results
Average baseline recession was 2.5 ± 1.0 mm. The surgery was 15.7 min shorter (95%CI from 11.9 to 19.6, p < .0001) and perceived lighter (11.9 VAS units, 95%CI from 4.6 to 19.1, p = .0014) in CMX subjects. Time to recovery was 1.8 days shorter in CMX. Six‐month root coverage was 1.7 ± 1.1 mm for CMX and 2.1 ± 1.0 mm for CTG (difference of 0.44 mm, 95%CI from 0.25 to 0.63 mm). The upper limit of the confidence interval was over the non‐inferiority margin of 0.25 mm. Odds of complete root coverage were significantly higher for CTG (OR = 4.0, 95% CI 1.8–8.8).
Conclusion
Replacing CTG with CMX shortens time to recovery and decreases morbidity, but the tested generation of devices is probably inferior to autologous CTG in terms of root coverage. Significant variability in PROMs was observed among centres.
Keywords: collagen matrix, coronally advanced flap, gingival recession, human, randomized controlled clinical trial, root coverage
Clinical Relevance.
Scientific rationale for the study: In the root coverage of multiple adjacent recessions, availability of donor site for autologous connective tissue harvesting and postoperative morbidity provides compelling rationale for the application of adjunctive devices.
Principal findings: The results of this trial indicated that avoidance of CTG and application of collagen matrix (CMX) provided a better patient experience and shortened the time to recovery after coronally advanced flaps for coverage of multiple adjacent recessions. The non‐inferiority hypothesis of CMX in terms of root coverage, however, was rejected and CTG resulted in improved odds of complete root coverage.
Practical implications: The clinical indications for the use of first‐generation CMX are primarily: (i) cases with contraindication to autologous CTG harvesting from the palate; (ii) cases where the patient and clinician are seeking to limit morbidity, shorten surgery and time to recovery and are willing to accept a higher chance of a less than optimal outcome in terms of root coverage.
1. INTRODUCTION
Gingival recessions affect a large proportion of patients. Their presence has both aesthetic and functional implications as they are frequently associated with loss of hard tooth substance in the cervical area and root sensitivity (Pini‐Prato, Cairo et al., 2010; Pini‐Prato, Franceschi, Cairo, Nieri, & Rotundo, 2010). Recognition that long‐term root exposure to the oral cavity leads to root wear, hypersensitivity and cervical/root caries has provided a new more medical dimension to periodontal plastic surgery (Tonetti & Jepsen, 2014). Recent systematic reviews on single‐tooth recession have indicated that coronally advanced flaps are the most effective surgical procedure and that the addition of either an autologous connective tissue graft or enamel matrix derivative increased the outcome of these interventions (Cairo, Nieri, & Pagliaro, 2014). In practice, the addition of a connective tissue graft under a coronally advanced flap is considered the standard of care for achieving complete root coverage of single‐tooth recessions. Besides achievement of complete root coverage, potential advantages of connective tissue grafting include an increase in marginal tissue thickness that, in turn, may enhance long‐term stability. The addition of a connective tissue graft, however, complicates the surgical procedure and increases the rates of wound failure during early healing.
Recent evidence has indicated that the presence and type of loss of hard tissue substance in the cervical region of the tooth is frequently associated with recession (Pini‐Prato, Cairo et al., 2010; Pini‐Prato, Franceschi et al., 2010) and that such abrasions and erosions impact on clinical outcomes (Pini‐Prato, Magnani, Zaheer, Rotundo, & Buti, 2015). In fact, several clinicians have proposed approaches to the reconstruction of the eroded cemento‐enamel junction and/or of the abraded root surface (Cairo and Pini‐Prato 2010, Zucchelli et al. 2011).
Frequently recessions affect multiple adjacent teeth, and recent surgical approaches have offered novel opportunities to manage these patients with coronally advanced flaps (Zucchelli & de Sanctis, 2000) or tunnel techniques (Aroca et al., 2013). Thanks to the development of specific techniques, increased attention has been paid to the management of multiple adjacent recession, but the evidence base is still comparatively small (Graziani et al., 2014). Of interest, however, are reports that indicate that it is possible to simultaneously manage multiple adjacent recessions and that specific flap designs may offer selective advantages.
Clinical management of multiple adjacent recessions poses unique challenges and one relates to limitations in the size, shape and homogeneous thickness of autologous connective tissue grafts that can be harvested from the palate. Several studies have focused on alternatives including human dermis and collagen matrices (for review, please see Cairo et al. (2014)). The latter option includes potential advantages as: (i) it does not need human donors; (ii) there are no risks of transmission of human diseases; (iii) it is regulated as a medical device rather than as a transplant; and (iv) there are no limits in size of the surgical site. Experimental animal studies have indicated that xenogenic collagen matrix (CMX) is replaced with the host own tissue with the desired histologic and functional characteristics (Thoma, Villar, Cochran, Hammerle, & Jung, 2012) and leads to an augmentation in both the width and the thickness of the band of keratinized tissue (Thoma et al., 2011; Vignoletti et al., 2011; Thoma et al., 2010).
A pilot study on isolated recession indicated that combining CMX with a coronally advanced flap led to good clinical outcomes that compared favourably with those obtained with the use of autologous connective tissue grafts (McGuire & Scheyer, 2010). A more recent multicentre trial in isolated recessions indicated that the addition of CMX to coronally advanced flap resulted in a significant benefit in terms of marginal soft tissue thickness and patient‐reported outcomes compared to coronally advanced flaps alone (Stefanini et al., 2016).
This study was designed to test two hypotheses combining non‐inferiority testing of root coverage with superiority testing of patient‐reported outcomes. The first objective was to assess whether, in subjects with multiple adjacent gingival recessions treated with coronally advanced flaps, placement of xenogenic collagen matrix was non‐inferior compared to the combination with autologous connective tissue graft in terms of millimetres of root coverage (primary outcome), complete root coverage, wound healing and professional evaluation of aesthetics. The second objective of this study was to assess whether the addition of CMX to coronally advanced flap was superior in reducing surgical time and morbidity, shortening time to recovery and improving patient‐reported outcomes with respect to the use of autologous connective tissue graft used to obtain root coverage of multiple adjacent gingival recessions. This manuscript deals with patient‐reported outcomes and root coverage outcomes. A further manuscript is planned to report on aesthetics and wound healing outcomes.
2. MATERIALS AND METHODS
2.1. Study design and population
This was a randomized, controlled, parallel arm, standard of care‐controlled, assessor‐blind, multicentre, multinational, practice‐based trial with 6‐month follow‐up (clincialtrial.gov registration NCT01440426). The design tested two hypotheses, with a non‐inferiority trial in terms of the professional outcomes measuring root coverage and aesthetics and a superiority trial in terms of patient‐based outcomes. Primary ethical approval was obtained by the Freiburg Ethic Committee International (FEKI code 011/1546) and by the competent local authority for each centre. All subjects gave informed consent, and all study procedures were performed according to the Declaration of Helsinki on experimentation involving human subjects. Individuals, (i) with a minimum of two adjacent recessions of the gingival margin (of which at least one 3 mm or deeper) requiring surgical intervention for root coverage, (ii) no prior experience of root coverage procedures and (iii) able to achieve good oral hygiene and control gingivitis in the whole of the dentition (FMPS < 25% and FMBS < 25%), were invited to participate. Exclusion criteria comprised: (i) untreated periodontitis; (ii) persistence of uncorrected gingival trauma from toothbrushing; (iii) inter‐dental attachment loss greater than 1 mm or furcation involvement in the teeth to be treated; (iv) presence of severe tooth malposition, rotation or clinically significant super‐eruption; (v) self‐reported current smoking exceeding 20 cigarettes/day or pipe or cigar smoking; (vi) rheumatoid arthritis or known sensitization to collagen‐based medical products, and/or presence of medical contraindications to elective surgery. Indications for surgical intervention were established based on patient's concerns with aesthetic, root sensitivity, brushing, root abrasion and/or caries development by patient interview and clinical examination. Prior therapy included treatment of gingivitis, if present, instructions in non‐traumatic oral hygiene and reconstruction of the cemento‐enamel junction (CEJ) with adhesive dental materials in case of cervical abrasion or erosion involving this structure. Trial recruitment occurred between January 2012 and December 2013. Six‐month follow‐up was completed by July 2014.
2.2. Control of study bias
A central study registrar based at the ERGOPerio clinical research infrastructure in Genova, Italy, performed study registration and treatment assignment procedures. Subjects were randomized to test or control treatment based on computer‐generated random codes using random permuted blocks with a block size of 4 and minimization for cigarette smoking (Tonetti et al., 1998; Cortellini et al. 2001). Allocation was concealed to the surgeon by opaque envelopes to be opened upon completion of the common portion of the surgery, that is preparation of the recipient bed for the graft or the collagen matrix. Study personnel administering questionnaires and clinical examiners were masked with respect to treatment allocation. All investigators participated in a 3‐day calibration meeting. Examiners had to achieve an intra‐examiner reproducibility >98% within 1 mm for the distance between the incisal margin and the gingival margin in 10 non‐study‐related subjects with gingival recessions (Cairo et al., 2016). The surgical procedure was standardized through discussions of specifically produced videos.
2.3. Interventions
The two intervention groups consisted of coronally advanced flaps with autologous connective tissue graft (standard of care control) or a xenogeneic collagen matrix (Geistlich Mucograft®, Geistlich Pharma AG, Wolhusen, Switzerland). Coronally advanced flaps were designed based on the location and distribution of the recessions and included either rotated papillae flap or trapezoidal flap designs with or without vertical releasing incisions (Cairo et al., 2016; Cortellini et al., 2009; Zucchelli & de Sanctis, 2000). Whenever applicable the rotated papillae flap was preferred. Split‐full‐split thickness flaps were elevated with an M6400 mini‐micro surgical blade (Keydent, Bologna, Italy) as follows: inter‐dental papillae were prepared split thickness, and marginal soft tissue was preserved by full thickness elevation of the buccal portion of the flap until the mucogingival junction, apical extension of the flap was achieved split thickness. Periosteal incision or vertical releasing incisions were aimed at the coronal advancement of the flap in the absence of flap tension. The randomization envelope was opened after completion of the preparation of the recipient bed of the graft. Autologous connective tissue grafts were harvested from the palate as previously described using the trap door technique whenever feasible and the de‐epithelialized free gingival graft in cases with insufficient tissue thickness (Cairo et al., 2016). Grafts were stabilized 1 mm apical to the cement–enamel junction with 6‐0 braided resorbable polylactic sutures. Dried CMX was adapted and sutured in the same way in the test sites. Flaps were sutured with interrupted (Seralene, Serag and Wiessner, Germany) and/or sling (e‐PTFE, W.L. Gore, Arizona, USA) 6‐0 and 7‐0 monofilament sutures attempting to fully cover both CTG and CMX. Sutures were removed after 7‐10 days. Pain control consisted of 600 mg ibuprofen or 500 mg paracetamol; patients were instructed to take one tablet at the end of the procedure and one 6 hours later and to continue as needed in case of pain. Antibiotics were prescribed, as needed, at discretion of the single clinician. The postsurgical follow‐up consisted of modified oral hygiene in the treated area, chlorhexidine mouth rinsing and professional prophylaxis with 0.5% chlorhexidine gel. At each centre, a single clinician experienced in periodontal plastic surgery performed all surgeries (MA, FC, GC, YF, FG, AG, JH, GR, HT, HW, BW, IZ and OZ).
2.4. Clinical measures
The position of the gingival margin was measured to the nearest mm with a UNC15 periodontal probe (PCP‐UNC 15; Hu‐Friedy, Chicago, USA) using both the incisal edge and the natural or composite filling reconstructed cement–enamel junction (CEJ) as the reference point. Outcomes were assessed using changes in recession from the incisal edge as the reference; recessions were characterized using the CEJ as the reference. Depth of the gingival sulcus (PD) and width of the keratinized tissue (KT) were assessed clinically with a UNC15 probe. The location of the mucogingival junction was assessed with the visual and functional method and supplemented by the histochemical method in areas of unclear demarcation (Guglielmoni, Promsudthi, Tatakis, & Trombelli, 2001). Oral hygiene levels were assessed with the plaque control record (O'Leary 1972), while gingival inflammation was assessed as percentage of sites with bleeding on probing (Tonetti et al. 1993).
2.5. Patient‐reported outcomes
Patient concerns with gingival recession were assessed with a condition‐specific health‐related quality‐of‐life instrument assessing the level of concern in terms of aesthetics, sensitivity to cold, sensitivity to brushing, root/tooth wear, development of cavity and fear to lose the involved teeth using a questionnaire on a 5‐point Likert scale at baseline and 6 months (Appendix S1). Perceptions of oral health‐related quality of life were assessed using validated translations of the OHIP‐14 questionnaire into the native language of all participants. Patients were instructed to use a postoperative diary for the first 14 days after the surgery to capture patient‐reported experience measures (PREMs). The diary was designed to assess patient recovery in four main areas: postsurgery sequelae, pain and discomfort, oral function, interference with daily activities (Appendix S2). All questionnaires had been piloted in two centres on patients undergoing root coverage and had been shown to capture patient concerns. Patient perceptions of pain and discomfort were rated using a VAS. Time to recovery was calculated as the time required for OHIP values to be equal or lower than the baseline ones and as the time necessary to reach a VAS <10 in terms of pain.
2.6. Dentine hypersensitivity
Root sensitivity was assessed with the air blow test and the Yeaple probe test as previously described for gingival recession sites (Cortellini et al., 2009).
2.7. Assessment of early wound healing
Early wound healing was assessed at the 1‐, 2‐, 4‐ and 12‐week follow‐up appointment using a modification of the early wound healing index (m‐EHI; Wachtel et al., 2003) assessing healing of the flap margin (area of original recession), the inter‐dental papillae and the graft. Healing of the flap margin was evaluated with reference to the CEJ or margin of the restoration in terms of presence of swelling/inflammation, presence of fibrin accumulation/tissue necrosis and presence of obvious mobility of the wound margin on the root surface. With regard to the papillae, the primary union between the flap margin and the papillae and presence of fibrin or an area of necrosis in the papilla area were evaluated. Wound healing of the graft was visually assessed in terms of its exposure underneath the flap, apparent mobility of the graft and presence of areas of necrosis of the flap, if exposed. Presence of all sutures and their slack was assessed at the 1‐week follow‐up.
2.8. Sample size
Given the larger sample size required for the non‐inferiority hypothesis, the study has been sized on recession coverage. The non‐inferiority margin in a decrease in a gingival recession was defined as half the clinically relevant difference and set at 0.25 mm. Sample size calculations were based on a pilot study including 6 centres, 46 patients and 186 recessions showing a recession reduction of 1.63 ± 1.12 mm, a root mean square error of 0.85 mm using baseline recession as a covariate and an intraclass correlation coefficient (recessions nested into patients) of 0.34. Ninety‐two subjects with four adjacent recessions per treatment arm were required to have 80% power with alpha set at 0.025 to be consistent with 95% confidence interval of non‐inferiority trial.
2.9. Statistical analysis
Data were entered into a database and proofed for entry errors. Descriptive statistics were summarized as means and standard deviations for quantitative data and frequencies and percentages for qualitative data. Multilevel analyses were performed with the treatment (CMX versus CTG) as explicative variable. For the site outcome variables (e.g. root coverage), the three levels of the models were centre, patient and site. Baseline values were used as a covariate. For complete root coverage, a three‐level logistical model was tested using CEJ‐GM at baseline as a covariate. For the patient outcome variables (e.g. VAS), the two levels of the models were centre and patients. The intraclass correlation coefficients were calculated to estimate the variability among centres. Estimates for the treatment effect, standard errors and 95% confidence intervals were provided. The statistical software was MLwiN 2.21 Centre for Multilevel Modelling, University of Bristol, UK, and JMP 13.0.0; SAS Institute Inc., Cary, NC, USA.
3. RESULTS
3.1. Study population and external validity
Figure 1 shows the CONSORT patient accountability; 187 subjects with recession at 485 teeth were randomized and received the allocated intervention. With the exception of 2 post‐op diaries that were misplaced, all subjects completed the 6‐month follow‐up. Table 1 shows the patient characteristics. Table 2 reports the local condition at teeth with recession that were included. Data on the presence of cervical fillings, cervical caries, CEJ abrasion and root step as well as their pre‐treatment before moving into the surgical procedure indicate that the CMX group presented more frequently with a cervical filling or a cervical step on the exposed root surface. The locations of the treated teeth for the test and control groups are presented in Table S1 (Appendix S3).
Figure 1.
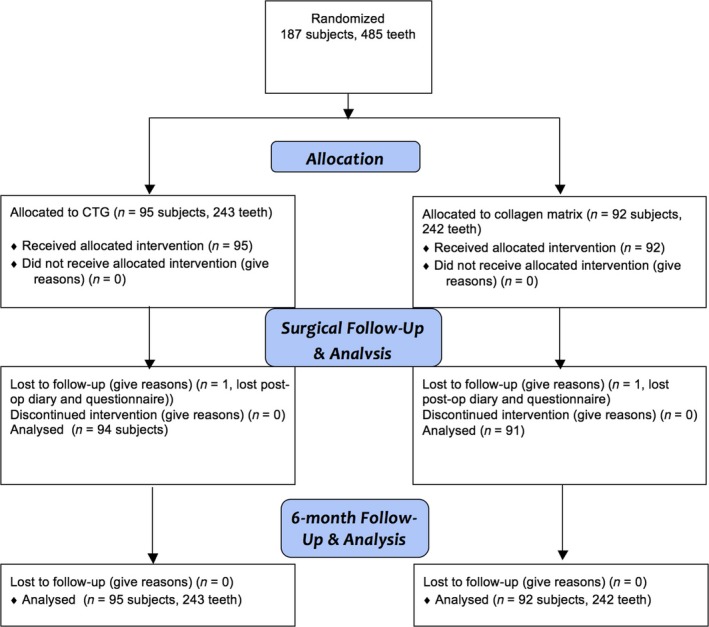
CONSORT patient accountability diagram
Table 1.
Study population
| CTGN = 95 | CMXN = 92 | |
|---|---|---|
| Age (years) | 39.0 ± 10.5 | 41.3 ± 10.0 |
| Females (F) | 61 (64%) | 57 (62%) |
| Smokers | 15 (16%) | 16 (17%) |
| Baseline OHIP‐14 values | 10.0 ± 6.9 | 9.7 ± 7.1 |
| Full‐mouth plaque scores | 12.1 ± 7.6 | 12.9 ± 8.8 |
| Full‐mouth bleeding score | 7.0 ± 6.9 | 6.7 ± 5.8 |
| Dentine sensitivity air test positive | 61 (64%) | 58 (63%) |
| Dentine sensitivity Yeaple test positive | 32 (34%) | 44 (48%) |
Patient‐level baseline characteristics (mean ± SD or frequency and percentage).
FMPS, full‐mouth plaque score; FMBS, full‐mouth bleeding score.
Table 2.
Baseline characteristics of teeth with recessions
| Variable | CTGN = 243 | CMXN = 242 |
|---|---|---|
| Distance from CEJ‐GM (gingival margin) mm | 2.5 ± 1.0 | 2.5 ± 1.0 |
| Distance from incisal edge to GM mm | 11.7 ± 1.6 | 11.9 ± 2.0 |
| Probing depth mm | 1.5 ± 0.5 | 1.5 ± 0.6 |
| Width of keratinized tissue mm | 2.9 ± 1.3 | 3.0 ± 1.4 |
| Max inter‐dental clinical attachment loss mm | 0.3 ± 0.5 | 0.3 ± 0.6 |
| Presence of inter‐dental clinical attachment loss | 62 (26%) | 67 (28%) |
| Local plaque score | 0 (0%) | 3 (1%) |
| Local bleeding on probing | 0 (0%) | 0 (0%) |
| Cervical filling | 15 (6%) | 37 (15%) |
| Cervical filling removed, if present | 10 (67%) | 26 (70%) |
| Cervical caries | 18 (7%) | 23 (9%) |
| Cervical caries treated, if caries present | 18 (100%) | 23 (100%) |
| Cervical caries filled, if caries present | 18 (100%) | 16 (70%) |
| Presence of CEJ abrasion | 97 (40%) | 103 (43%) |
| CEJ abrasion reconstructed with adhesive reconstruction, if present | 85 (88%) | 86 (83%) |
| Presence of cervical step | 46 (19%) | 82 (34%) |
| Cervical step rounded, if present | 24 (52%) | 40 (49%) |
| Cervical step reconstructed with adhesive reconstruction, if present | 22 (48%) | 48 (59%) |
Means ± SD or frequency (percentage).
3.2. Surgical outcomes
Table 3 shows the description of the surgery performed in the two groups. CTG surgery lasted 16 minutes longer than CMX. An important centre effect (high percentage of intraclass correlation coefficient) was observed for the duration of the surgery. All other surgical parameters were similar with the exception that the corono‐apical width of the CMX graft was larger than for the CTG. The flap was extended horizontally for 2.0 ± 1.3 and 1.7 ± 1.1 teeth, and mesial vertical releasing incisions were placed in 16% and 13% of cases, while distal vertical releasing incisions were placed in 20% and 16% of cases treated with CTG and CMX, respectively. Parameters assessing the technical outcome of the surgery showed a very high degree of success for both groups: the CEJ was covered by the CAF in 99.6% and 100% of teeth, the graft was completely covered in 96% and 98% of teeth, and primary wound closure was achieved in 98% and 99.6% of teeth for CTG and CMX, respectively.
Table 3.
Patient‐based surgical parameters and patient perceptions during the surgery and tooth‐based surgical parameters
| Variable | CTGN = 94 patients | CMXN = 91 patients | Estimated difference (95% CI) | p‐value | ICC% |
|---|---|---|---|---|---|
| Duration of surgery (min) | 69.7 ± 24.3 | 53.2 ± 17.7 | 15.7 (11.9–19.6) | <.0001 | 61.7% |
| Patient perception of hardship of procedure (VAS) | 41.5 ± 26.7 | 29.5 ± 26.4 | 11.9 (4.6–19.1) | .0014 | 11.5% |
| Patient perception of pain during the procedure (VAS) | 11.6 ± 17.6 | 9.2 ± 14.1 | 2.2 (−6.7–2.2) | .3266 | 7.5% |
| Patient preference for alternate therapy at end of surgery | 44 ± 32.7 | 27.4 ± 27.4 | 16.6 (7.9–25.3) | .0002 | 0.8% |
| N = 243 teeth | N = 242 teeth | ||||
|---|---|---|---|---|---|
| Distance from CEJ‐to coronal margin of graft (mm)a | 0.2 ± 0.6 | 0.3 ± 0.7 | −0.06 (−0.24; 0.11) | .4777 | |
| Apico‐coronal height of graft (mm)b | 5.3 ± 1.5 | 7.6 ± 2.3 | −2.2 (−2.6; −1.7) | <.0001 |
Unless otherwise specified, data are means ± SD. ICC, intraclass correlation coefficient.
N = 238 for CTG and N = 235 per CMX.
N = 240 for CTG and N = 235 for CMX additional data are presented in the text.
Patient randomized to CTG surgery perceived greater hardship during the procedure (with a moderate centre effect).
With regard to CTG harvesting, surgeons performed the trap door technique in 58 cases and the de‐epithelialized free gingival graft in the remaining 37. The harvested grafts were 19.6 ± 6.6 mm in length and 5.3 ± 1.5 mm in height. Additional parameters for the CTG harvesting site and its healing progression are reported in Table S2 (Appendix S3).
3.3. Time to recovery
The detailed patient‐reported outcomes based on the postsurgical diaries are illustrated in Table 3 (Appendix S3). OHIP‐14 scores were significantly elevated in the first days after surgery indicating the good sensitivity of the instrument for postsurgical monitoring of these procedures. The time to recovery in terms of OHIP scores was 6.0 ± 4.1 days in the CTG groups and 4.2 ± 3.4 days in the CMX group. The 1.8‐day difference was statistically significant. With regard to pain experience assessed on a VAS, the average time to no significant pain (values <10 on the VAS scale) was 3 to 4 days and the time to no pain (VAS = 0) was 8 to 9 days. Patients in the CMX group perceived less pain at 7 days. No treatment differences in pain were observed at other times. Subjects in the CTG group took analgesic medications for one extra day compared to the CMX. A large centre effect was observed for OHIP scores 1 day after surgery and for the duration of pharmacological pain control. Daily value of average pain experience and OHIP over the first 14 days is illustrated in Figure 2a, b, respectively.
Figure 2.
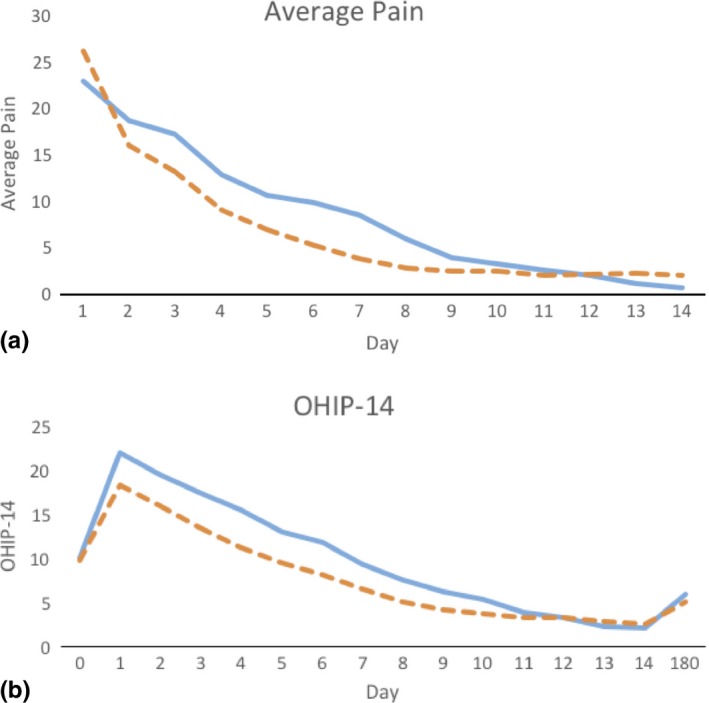
Perception of pain and OHIP‐14 scores after surgery. Average postoperative pain values (a) and OHIP‐14 scores (b) representative of the previous 24 hr during the first 14 days after surgery in subjects treated with CTG (blue continuous line) and CMX (orange dashed line). Pain values are expressed on a 100‐mm visual analogue scale (values under 30 are considered to be moderate pain). Intergroup differences for pain were significant at 7 days. OHIP‐14 scores are expressed as total scores (values ranging from 0 to 56, with higher scores indicating greater impact). OHIP scores were lower in CMX patients at days 1, 3 and 7. The time necessary to return to baseline OHIP scores (time to recovery) was 1.8 days shorter in CMX‐treated subjects
3.4. Early wound healing
Tooth‐level wound healing parameters observed at the 1‐, 2‐ and 4‐week postoperative follow‐up are displayed in Table S4 (Appendix S3) in terms of complete root coverage, flap margin, graft and papillae.
3.5. Root coverage
The progression of healing in terms of complete root coverage is displayed in Figure 3. At the completion of surgery, the CEJ was covered in 242 teeth for both groups (in one tooth in the CTG group, it was not possible to cover the CEJ). Over time, contraction of the flap margin led to the exposure of the CEJ in an increasing number of cases. Odds ratios of complete root coverage at 6 months were significantly higher for CTG than CMX‐treated cases (Table 4). The results of the multilevel model estimating treatment differences in terms of root coverage (distance from the CEJ and the incisal edge to the gingival margin) are displayed in Table 4. The 95% CI of the treatment difference at 6 months was 0.25; 0.63 and the upper limit of the confidence interval are over the non‐inferiority margin of 0.25 mm. The non‐inferiority hypothesis cannot be rejected, and the superiority of CTG over CMX cannot be excluded. Similar results were observed using the incisal edge as the reference point to determine changes in the position of the gingival margin. Statistically significant but clinically irrelevant changes in PD were observed. In terms of changes in KT, the CTG group gained 0.5 mm, while the CMX group lost 0.1 mm (estimated difference 0.47 mm, 95% CI 0.24 to 0.70).
Figure 3.
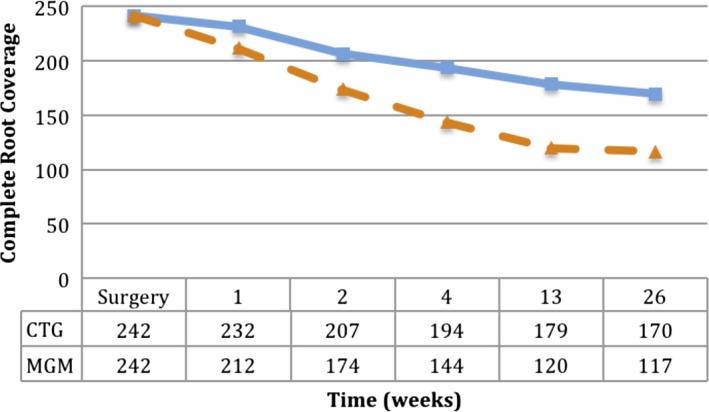
Number of teeth with complete root coverage over time. Number of teeth with complete root coverage at different time points in subjects treated with CTG (blue continuous line) and CMX (orange dashed line)
Table 4.
Six‐month clinical outcomes. Multilevel model: centre, patient, tooth
| Variable | CTG N = 243 | CMX N = 242 | Estimated difference (odds ratio*) | 95% CI |
|---|---|---|---|---|
| Changes in CEJ‐GM (mm) | 2.1 (1.0) | 1.7 (1.1) | 0.44 | 0.25; 0.63 |
| Changes in IE‐GM (mm) | 2.1 (1.1) | 1.4 (1.2) | 0.63 | 0.43; 0.84 |
| Changes in PD (mm) | −0.3 (0.8) | −0.1 (0.7) | −0.18 | −0.31; −0.04 |
| Changes in KT (mm) | 0.5 (1.2) | −0.1 (1.1) | 0.47 | 0.24; 0.70 |
| Complete root coverage N (%) | 170 (70%) | 117 (48%) | 4.02* | 1.83; 8.83 |
Multilevel model estimating clinical outcomes taking into account clustering of multiple sites in a single patient (surgery) and patients within a specific study centre. Changes in CEJ‐GM = changes in the distance from the cemento‐enamel junction to the gingival margin. Changes in IE‐GM (mm) = changes in the distance between the incisal edge of the tooth and the gingival margin. They are estimates of root coverage. PD, probing depth; KT, width of keratinized tissue. Data are expressed as means (SD) in mm.
Improvements in the frequency of dentine sensitivity were observed in both groups. Patients treated with CMX had 2.96 higher OR of displaying dentine sensitivity to the air blow test than CTG subjects. Results were not confirmed with the Yeaple probe test (Table S5, Appendix S3).
Changes in condition‐specific OHRQoL scores are reported in Table S6 (Appendix S3). Improvements in all parameters were observed comparing baseline to the 6‐month follow‐up. Averages indicated changes from the “some concern” and “quite a bit of concern” (2–3) Likert scores to the “little concern” score (1). No treatment‐associated changes were observed.
4. DISCUSSION
The results of this trial provide strong evidence that the adjunct of a xenogeneic collagen matrix to coronally advanced flaps for coverage of multiple adjacent recessions results in shorter surgical time, shorter time to recovery and better patient perception compared to autologous connective tissue graft. The results, however, failed to support the non‐inferiority hypothesis of CMX with respect to CTG in terms of root coverage; the position of the non‐inferiority margin with respect to the 95% confidence interval suggests that CTG addition to coronally advanced flaps may be superior to CMX. These data, combined with the results of a recent multicentre trial indicating that the addition of CMX to coronally advanced flaps provides a benefit in terms of root coverage outcomes in isolated recessions (Stefanini et al., 2016), suggest that the clinical indications for the use of CMX are primarily: (i) cases with contraindication to autologous CTG harvesting from the palate; (ii) cases where the patient and clinician are seeking to limit morbidity and are willing to accept a higher chance of a less than optimal outcome. Systematic reviews indicate that an adjunct to the coronally advanced flap results in better outcomes both in single and multiple recessions (Cairo et al., 2014; Graziani et al., 2014). The recent trial by Cairo et al. (2016) provides further insight: the thickness of the flap margin seems to modify the short‐term benefit from the adjunct of CTG to coronally advanced flap. The adjunctive application of CMX or CTG to coronally advanced flaps may therefore add a benefit at recession sites with thinner marginal tissues. These data support clinical insight that limits the application of CTG or devices to thinner tissues after a site‐specific assessment (Cortellini & Pini Prato, 2012). Longer‐term studies, however, to support this selective approach are lacking: available data indicate that thickening of the coronally advanced flap with CTG provides better stability of the root coverage outcome compared to CAF alone (Pini‐Prato, Cairo et al., 2010). Only limited data are available about changes in marginal tissue thickness after adjunctive use of CMX (Cardaropoli, Tamagnone, Roffredo, & Gaveglio, 2012; Jepsen et al., 2013).
Recent reports addressing a novel generation of collagen matrices with improved volumetric stability during the healing period are relevant to the interpretation of the current results. Preclinical studies with such devices point to similar increases in soft tissue thickness at dental implant sites treated with CTG and newer generation collagen matrices (Thoma, Naenni, Benic, Hammerle, & Jung, 2017). Results have been confirmed in short‐term pilot trials and point to the potential of second‐generation matrices to yield clinically equivalent outcomes compared with autologous CTG (Thoma, Zeltner et al., 2016; Zeltner, Jung, Hammerle, Husler, & Thoma, 2017).
The present short‐term root coverage data need to be interpreted with a degree of caution in the light of initial longer‐term reports suggesting better 5‐year stability with CTG compared with CMX at single‐tooth recessions (McGuire & Scheyer, 2016) and similar degrees of 3‐year stability with both coronally advanced flap and adjunctive CMX at single (isolated) recessions (Jepsen, Stefanini, Sanz, Zucchelli, & Jepsen, 2017).
This trial describes postoperative time to recovery in periodontology using patient‐reported outcomes. Results showed changes in OHIP‐14 during the postoperative period after plastic periodontal surgery. An earlier increase was followed by return towards baseline values within the first two postoperative weeks. Using baseline OHIP‐14 values, it is therefore possible to estimate the time to recovery after periodontal plastic surgery. In this trial, the major patient‐reported benefits of the use of CMX were to: (i) shorten time to recovery by 1.8 days; (ii) require 1 day less of pain control medications, compared to CTG. These benefits may be expected from the avoidance of graft harvesting from the palate. In previous studies, we have used questionnaires and visual analogue scales to assess patient perceptions of the postoperative period and estimate differences between different surgical modalities (Tonetti et al., 1998, 2004; Lang et al., 2007; Tonetti et al., 2017; Cortellini & Tonetti, 2007). Similar approaches have been used in recent studies assessing postoperative morbidity of periodontal surgery (Tan, Krishnaswamy, Ong, & Lang, 2014; Mei, Lee, & Yeh, 2016). A recent commentary raised awareness of the reliability, validity, sensitivity and clinical relevance issues with the use of current approaches to study postoperative morbidity and recovery in periodontal surgery (McGuire, Scheyer, & Gwaltney, 2014). These results are an important step forward in developing better approaches and are in general agreement with the findings reported off‐protocol in the mentioned commentary. In periodontal plastic surgery procedures, a substantial portion of morbidity is attributed to postoperative pain consequent to harvesting of soft tissues grafts from the palate. The results of this study support only in part this notion: significant treatment‐associated differences in OHIP‐14 were observed at 1, 3 and 7 days after surgery, while a significant difference in pain evaluated by a visual analogue scale was observed only at day 7. Furthermore, at the earlier time points, the treatment‐associated effect size in OHIP‐14 represents only 15% of the magnitude of the time associated changes. These data suggest that the recipient site may be the major contributor to patient‐reported outcomes during early healing. Recent trials focusing on healing of palatal donor sites seem to support this hypothesis: they indicated that the size of the graft does not seem to be associated with postoperative pain and that parameters like the depth of the wound may be more important (Zucchelli et al., 2014; Burkhardt, Hammerle, & Lang, 2015). A planned companion report of this trial will explore this aspect in greater detail.
In this trial, the significance of the centre effect was assessed as intraclass correlation coefficient (ICC) of the multilevel data. For several parameters, the ICC was moderate to high. From a surgical standpoint, parameters affected by the centre effect were the length of the procedure with a high ICC, and the patient perception of the hardship of the procedure and the perception of pain during the procedure with a moderate ICC. Patient perception of the postoperative period was also affected by the centre effect: OHIP‐14 scores earlier on in the postoperative period, duration of pain and the length of both the analgesic and antibiotic treatment had moderate ICC. These data portray a significant effect of the individual surgeon on the patient experience of both the procedure and the postoperative recovery period with significant dimensions related to the length of the procedure, peri‐operative anxiety/discomfort/pain, time to recovery and postoperative recommendations.
This is the first large‐scale root coverage trial that enrolled a broad population of patients with recession defects combined with cervical hard tissue defects in a significant proportion of the population. The presence of hard tissue defects in the form of cervical abrasions, root caries and composite fillings has become a key characteristic of many areas with the gingival recession (Pini‐Prato, Franceschi et al., 2010) and in the absence of adequate management these impact clinical outcome (Pini‐Prato et al., 2015). This requires interdisciplinary management of the hard and soft tissue defects, and a variety of approaches have been proposed. It is particularly important to note that root surface characteristics and need for pre‐treatment were explicitly reported in this study. An exploratory analysis (data not shown) stratified by the baseline root surface conditions failed to show a difference in term of root coverage between pristine and compromised root surfaces/cervical areas. This is an important preliminary observation that points to external validity of the results to both populations. More research is needed in this area.
Several important conclusions can be drawn from this study: (i) there are clearly measurable patient benefits deriving from the avoidance of autologous soft tissue grafting by replacing them with collagen matrix‐based devices in multiple adjacent recessions; (ii) the tested generation of devices is probably inferior to adjunctive autologous connective tissue grafting in these cases; (iii) moderate to high intraclass correlation coefficients for patient‐reported outcomes point to a significant centre effect and the opportunity of improvement in patient experience from periodontal plastic surgery procedures.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflict of interest in connection with this article.
Supporting information
ACKNOWLEDGEMENTS
Authors wish to recognize the clinical staff of the participating centres for their excellent clinical work that made this study possible.
Tonetti MS, Cortellini P, Pellegrini G, et al. Xenogenic collagen matrix or autologous connective tissue graft as adjunct to coronally advanced flaps for coverage of multiple adjacent gingival recession: Randomized trial assessing non‐inferiority in root coverage and superiority in oral health‐related quality of life. J Clin Periodontol. 2018;45:78–88. https://doi.org/10.1111/jcpe.12834
Funding information
This study was sponsored by the European Research Group on Periodontology (ERGOPerio). The study was financially supported in part by an unrestricted grant from Geistlich Pharma AG, Switzerland. The employed regenerative materials were a gift from Geistlich Pharma AG, Switzerland. This research was initiated by the investigators who independently performed all phases of the study including protocol development, experimental procedures, data analysis, result interpretation and reporting.
REFERENCES
- Aroca, S. , Molnar, B. , Windisch, P. , Gera, I. , Salvi, G. E. , Nikolidakis, D. , & Sculean, A. (2013). Treatment of multiple adjacent Miller class I and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: A randomized, controlled clinical trial. Journal of Clinical Periodontology, 40, 713–720. https://doi.org/10.1111/jcpe.12112 [DOI] [PubMed] [Google Scholar]
- Burkhardt, R. , Hammerle, C. H. , Lang, N. P. , & Research Group on Oral Soft Tissue Biology & Wound Healing (2015). Self‐reported pain perception of patients after mucosal graft harvesting in the palatal area. Journal of Clinical Periodontology, 42, 281–287. https://doi.org/10.1111/jcpe.12357 [DOI] [PubMed] [Google Scholar]
- Cairo, F. , & Pini‐Prato, G. P. (2010). A technique to identify and reconstruct the cementoenamel junction level using combined periodontal and restorative treatment of gingival recession. A prospective clinical study. International Journal of Periodontics and Restorative Dentistry, 30(6), 573–581. [PubMed] [Google Scholar]
- Cairo, F. , Cortellini, P. , Pilloni, A. , Nieri, M. , Cincinelli, S. , Amunni, F. , … Tonetti, M. S. (2016). Clinical efficacy of coronally advanced flap with or without connective tissue graft for the treatment of multiple adjacent gingival recessions in the aesthetic area: A randomized controlled clinical trial. Journal of Clinical Periodontology, 43, 849–856. https://doi.org/10.1111/jcpe.2016.43.issue-10 [DOI] [PubMed] [Google Scholar]
- Cairo, F. , Nieri, M. , & Pagliaro, U. (2014). Efficacy of periodontal plastic surgery procedures in the treatment of localized facial gingival recessions. A systematic review. Journal of Clinical Periodontology, 41(Suppl 15), S44–S62. https://doi.org/10.1111/jcpe.12182 [DOI] [PubMed] [Google Scholar]
- Cardaropoli, D. , Tamagnone, L. , Roffredo, A. , & Gaveglio, L. (2012). Treatment of gingival recession defects using coronally advanced flap with a porcine collagen matrix compared to coronally advanced flap with connective tissue graft: A randomized controlled clinical trial. Journal of Periodontology, 83, 321–328. https://doi.org/10.1902/jop.2011.110215 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , & Pini Prato, G. (2012). Coronally advanced flap and combination therapy for root coverage. Clincial strategies based on scientific evidence and clincial experience. Periodontology 2000, 59, 158–184. https://doi.org/10.1111/prd.2012.59.issue-1 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , & Tonetti, M. S. (2007). Minimally invasive surgical technique and enamel matrix derivative in intra‐bony defects. I: Clinical outcomes and morbidity. Journal of Clinical Periodontology, 34, 1082–1088. https://doi.org/10.1111/cpe.2007.34.issue-12 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Tonetti, M. , Baldi, C. , Francetti, L. , Rasperini, G. , Rotundo, R. , … Prato, G. P. (2009). Does placement of a connective tissue graft improve the outcomes of coronally advanced flap for coverage of single gingival recessions in upper anterior teeth? A multi‐centre, randomized, double‐blind, clinical trial. Journal of Clinical Periodontology, 36, 68–79. https://doi.org/10.1111/cpe.2009.36.issue-1 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Tonetti, M. S. , Lang, N. P. , Suvan, J. E. , Zucchelli, G. , Vangsted, T. , … Adriaens, P. (2001). The simplified papilla preservation flap in the regenerative treatment of deep intrabony defects: Clinical outcomes and postoperative morbidity. Journal of Periodontology, 72(12), 1702–1712. [DOI] [PubMed] [Google Scholar]
- Graziani, F. , Gennai, S. , Roldan, S. , Discepoli, N. , Buti, J. , Madianos, P. , & Herrera, D. (2014). Efficacy of periodontal plastic procedures in the treatment of multiple gingival recessions. Journal of Clinical Periodontology, 41(Suppl 15), S63–S76. https://doi.org/10.1111/jcpe.12172 [DOI] [PubMed] [Google Scholar]
- Guglielmoni, P. , Promsudthi, A. , Tatakis, D. N. , & Trombelli, L. (2001). Intra‐ and inter‐examiner reproducibility in keratinized tissue width assessment with 3 methods for mucogingival junction determination. Journal of Periodontology, 72, 134–139. https://doi.org/10.1902/jop.2001.72.2.134 [DOI] [PubMed] [Google Scholar]
- Jepsen, K. , Jepsen, S. , Zucchelli, G. , Stefanini, M. , de Sanctis, M. , Baldini, N. , … Sanz, M. (2013). Treatment of gingival recession defects with a coronally advanced flap and a xenogeneic collagen matrix: A multicenter randomized clinical trial. Journal of Clinical Periodontology, 40, 82–89. https://doi.org/10.1111/jcpe.2012.40.issue-1 [DOI] [PubMed] [Google Scholar]
- Jepsen, K. , Stefanini, M. , Sanz, M. , Zucchelli, G. , & Jepsen, S. (2017). Long‐term stability of root coverage by coronally advanced flap procedures. Journal of Periodontology, 88, 626–633. https://doi.org/10.1902/jop.2017.160767 [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , Tonetti, M. S. , Suvan, J. E. , Bernard, J. P. , Botticelli, D. , Fourmousis, I. , … Weber, H. P. (2007). Immediate implant placement with transmucosal healing in areas of aesthetic priority: A multicentre randomized‐controlled clinical trial I. Surgical outcomes. Clinical Oral Implants Research, 18, 188–196. https://doi.org/10.1111/clr.2007.18.issue-2 [DOI] [PubMed] [Google Scholar]
- McGuire, M. K. , & Scheyer, E. T. (2010). Xenogeneic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence‐type recession defects. Journal of Periodontology, 81, 1108–1117. https://doi.org/10.1902/jop.2010.090698 [DOI] [PubMed] [Google Scholar]
- McGuire, M. K. , & Scheyer, E. T. (2016). Long‐term results comparing xenogeneic collagen matrix and autogenous connective tissue grafts with coronally advanced flaps for treatment of dehiscence‐type recession defects. Journal of Periodontology, 87, 221–227. https://doi.org/10.1902/jop.2015.150386 [DOI] [PubMed] [Google Scholar]
- McGuire, M. K. , Scheyer, E. T. , & Gwaltney, C. (2014). Commentary: Incorporating patient‐reported outcomes in periodontal clinical trials. Journal of Periodontology, 85, 1313–1319. https://doi.org/10.1902/jop.2014.130693 [DOI] [PubMed] [Google Scholar]
- Mei, C. C. , Lee, F. Y. , & Yeh, H. C. (2016). Assessment of pain perception following periodontal and implant surgeries. Journal of Clinical Periodontology, 43, 1151–1159. https://doi.org/10.1111/jcpe.2016.43.issue-12 [DOI] [PubMed] [Google Scholar]
- O'Leary, T. J. , Drake, R. B. , & Naylor, J. E. (1972). The plaque control record. Journal of Periodontology, 43(1), 38. [DOI] [PubMed] [Google Scholar]
- Pini‐Prato, G. P. , Cairo, F. , Nieri, M. , Franceschi, D. , Rotundo, R. , & Cortellini, P. (2010). Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: A split‐mouth study with a 5‐year follow‐up. Journal of Clinical Periodontology, 37, 644–650. https://doi.org/10.1111/cpe.2010.37.issue-7 [DOI] [PubMed] [Google Scholar]
- Pini‐Prato, G. , Franceschi, D. , Cairo, F. , Nieri, M. , & Rotundo, R. (2010). Classification of dental surface defects in areas of gingival recession. Journal of Periodontology, 81, 885–890. https://doi.org/10.1902/jop.2010.090631 [DOI] [PubMed] [Google Scholar]
- Pini‐Prato, G. , Magnani, C. , Zaheer, F. , Rotundo, R. , & Buti, J. (2015). Influence of inter‐dental tissues and root surface condition on complete root coverage following treatment of gingival recessions: A 1‐year retrospective study. Journal of Clinical Periodontology, 42, 567–574. https://doi.org/10.1111/jcpe.2015.42.issue-6 [DOI] [PubMed] [Google Scholar]
- Stefanini, M. , Jepsen, K. , De Sanctis, M. , Baldini, N. , Greven, B. , Heinz, B. , … Zucchelli, G. (2016). Patient‐reported outcomes and aesthetic evaluation of root coverage procedures: A 12 months follow‐up of a randomized controlled clinical trial. Journal of Clinical Periodontology, 43, 1132–1141. https://doi.org/10.1111/jcpe.2016.43.issue-12 [DOI] [PubMed] [Google Scholar]
- Tan, W. C. , Krishnaswamy, G. , Ong, M. M. , & Lang, N. P. (2014). Patient‐reported outcome measures after routine periodontal and implant surgical procedures. Journal of Clinical Periodontology, 41, 618–624. https://doi.org/10.1111/jcpe.12248 [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Hammerle, C. H. , Cochran, D. L. , Jones, A. A. , Gorlach, C. , Uebersax, L. , … Jung, R. E. (2011). Soft tissue volume augmentation by the use of collagen‐based matrices in the dog mandible – a histological analysis. Journal of Clinical Periodontology, 38, 1063–1070. https://doi.org/10.1111/jcpe.2011.38.issue-11 [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Jung, R. E. , Schneider, D. , Cochran, D. L. , Ender, A. , Jones, A. A. , … Hammerle, C. H. (2010). Soft tissue volume augmentation by the use of collagen‐based matrices: A volumetric analysis. Journal of Clinical Periodontology, 37, 659–666. https://doi.org/10.1111/cpe.2010.37.issue-7 [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Villar, C. C. , Cochran, D. L. , Hammerle, C. H. , & Jung, R. E. (2012). Tissue integration of collagen‐based matrices: An experimental study in mice. Clinical Oral Implants Research, 23, 1333–1339. https://doi.org/10.1111/clr.2012.23.issue-12 [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Zeltner, M. , Hilbe, M. , Hammerle, C. H. , Husler, J. , & Jung, R. E. (2016). Randomized controlled clinical study evaluating effectiveness and safety of a volume‐stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. Journal of Clinical Periodontology, 43, 874–885. https://doi.org/10.1111/jcpe.2016.43.issue-10 [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Naenni, N. , Benic, G. I. , Hämmerle, C. H. , & Jung, R. E. (2017). Soft tissue volume augmentation at dental implant sites using a volume stable three‐dimensional collagen matrix – Histological outcomes of a preclinical study. Journal of Clinical Periodontology, 44(2), 185–194. https://doi.org/10.1111/jcpe.12635 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Cortellini, P. , Graziani, F. , Cairo, F. , Lang, N. P. , Abundo, R. , … Wetzel, A. (2017). Immediate versus delayed implant placement after anterior single tooth extraction: The timing randomized controlled clinical trial. Journal of Clinical Periodontology, 44, 215–224. https://doi.org/10.1111/jcpe.2017.44.issue-2 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Pini Prato, G. , Williams, R. C. , & Cortellini, P. (1993). Periodontal regeneration of human infrabony defects. III. Diagnostic strategies to detect bone gain. Journal of Periodontology, 64(4), 269–277. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Cortrllini, P. , Suvan, J. E. , Adriaens, P. , Baldi, C. , Dubravec, D. , … Lang, N. P. (1998). Generalizability of the added benefits of guided tissue regeneration in the treatment of deep intrabony defects. Evaluation in a multi‐center randomized controlled clinical trial. Journal of Periodontology, 69, 1183–1192. https://doi.org/10.1902/jop.1998.69.11.1183 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Fourmousis, I. , Suvan, J. , Cortellini, P. , Bragger, U. , Lang, N. P. , & European Research Group on Periodontology . (2004). Healing, post‐operative morbidity and patient perception of outcomes following regenerative therapy of deep intrabony defects. Journal of Clinical Periodontology, 31, 1092–1098. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Jepsen, S. , & Working Group 2 of the European Workshop on Periodontology (2014). Clinical efficacy of periodontal plastic surgery procedures: Consensus report of Group 2 of the 10th European Workshop on Periodontology. Journal of Clinical Periodontology, 41(Suppl 15), S36–S43. https://doi.org/10.1111/jcpe.12219 [DOI] [PubMed] [Google Scholar]
- Vignoletti, F. , Nunez, J. , Discepoli, N. , de Sanctis, F. , Caffesse, R. , Munoz, F. , … Sanz, M. (2011). Clinical and histological healing of a new collagen matrix in combination with the coronally advanced flap for the treatment of Miller class‐I recession defects: An experimental study in the minipig. Journal of Clinical Periodontology, 38, 847–855. https://doi.org/10.1111/jcpe.2011.38.issue-9 [DOI] [PubMed] [Google Scholar]
- Wachtel, H. , Schenk, G. , Böhm, S. , Weng, D. , Zuhr, O. , & Hürzeler, M. B. (2003). Microsurgical access flap and enamel matrix derivative for the treatment of periodontal intrabony defects: A controlled clinical study. Journal of Clinical Periodontology, 30, 496–504. https://doi.org/10.1034/j.1600-051X.2003.00013.x [DOI] [PubMed] [Google Scholar]
- Zeltner, M. , Jung, R. E. , Hammerle, C. H. , Husler, J. , & Thoma, D. S. (2017). Randomized controlled clinical study comparing a volume‐stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. Journal of Clinical Periodontology, 44, 446–453. https://doi.org/10.1111/jcpe.2017.44.issue-4 [DOI] [PubMed] [Google Scholar]
- Zucchelli, G. , & de Sanctis, M. (2000). Treatment of multiple recession‐type defects in patients with esthetic demands. Journal of Periodontology, 71, 1506–1514. https://doi.org/10.1902/jop.2000.71.9.1506 [DOI] [PubMed] [Google Scholar]
- Zucchelli, G. , Gori, G. , Mele, M. , Stefanini, M. , Mazzotti, C. , Marzadori, M. , … De Sanctis, M. (2011). Non‐carious cervical lesions associated with gingival recessions: A decision‐making process. Journal of Periodontology, 82(12), 1713–1724. https://doi.org/10.1902/jop.2011.110080 [DOI] [PubMed] [Google Scholar]
- Zucchelli, G. , Mounssif, I. , Mazzotti, C. , Montebugnoli, L. , Sangiorgi, M. , Mele, M. , & Stefanini, M. (2014). Does the dimension of the graft influence patient morbidity and root coverage outcomes? A randomized controlled clinical trial. Journal of Clinical Periodontology, 41, 708–716. https://doi.org/10.1111/jcpe.2014.41.issue-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


