Summary
The role of neuroimaging in the assessment of a first-ever seizure has not been well-defined, in particular the utility of MRI when CT is normal. The results of neuroimaging (CT brain, MRI brain, or both) in 1,013 adults with first-ever unprovoked seizure were correlated with clinical features and seizure outcome. Epileptogenic lesions were identified in 29%. Of patients with a normal CT who also had MRI, 12% had an epileptogenic lesion on MRI, the strongest independent predictor of which was a focal abnormality on EEG. Patients with an epileptogenic lesion had a higher risk of seizure recurrence, including when this was only evident on MRI.
There is general consensus that adults with an unprovoked first seizure should have brain imaging with either CT or MRI primarily to identify any process that may be responsible for the seizure.1–3 However, limited information is available on imaging findings in these patients. CT is generally readily available and excludes problems that require immediate attention in an acute setting,2 but MRI is the preferred imaging modality for patients with epilepsy because of its superior sensitivity for lesion detection.4 MRI may also be superior to CT in clarifying the electroclinical diagnosis, with an epileptogenic lesion supporting a focal seizure onset in an otherwise unclassified seizure.5 However, MRI may be unavailable or difficult to arrange within a clinically appropriate timeframe, and it is unclear whether MRI provides any additional prognostic information when CT is normal in patients with first-ever unprovoked seizure. Identifying factors predicting a positive MRI result when a CT is normal may be useful to prioritize patients for MRI. This study aims to determine the spectrum of findings on neuroimaging in patients with first-ever unprovoked seizure, to clarify the diagnostic and prognostic role of MRI when CT is normal, and to identify clinical features that predict positive MRI findings.
METHODS
Study population and investigations
Patients with first-ever unprovoked seizure presenting to a hospital-based adult epilepsy service in Perth, Western Australia, were prospectively identified between 2000 and 2011 as part of an ongoing first-ever seizure study.6 All patients underwent a standardized clinical assessment and the clinical, imaging, and EEG findings were prospectively entered into a database. The patients were drawn from a referral population of approximately 1.2 million people, mainly from metropolitan Perth, with most referred from emergency departments and the remainder from in-hospital referrals and general practitioners. Patients with prior seizures, nonepileptic events, and provoked seizures were excluded. Patients who had a second seizure while awaiting clinic review were included, with the second seizure defined as a recurrence. Seizure type and etiology were categorized for the presenting seizure according to published guidelines.7 Patients who did not have neuroimaging (CT, MRI, or both) were excluded. CT scans were performed at a variety of institutions (single helical–64 detector systems), usually scanned employing an axial technique with soft tissue reconstruction and multiplanar reformats. Contrast was administered if indicated by the clinical situation or an abnormality was detected. MRI scans were performed on a 1.0-T Picker (Marconi, Cleveland, OH), 1.5-T Siemens Sonata, or 1.5-T Siemens Vision system utilizing multiplanar T1, T2, and fluid-attenuated inversion recovery whole brain and dedicated temporal lobe imaging, also with contrast administered where clinically indicated. The majority of MRI scans were reported by a neuroradiologist. Not all patients had both CT and MRI, with the decision to proceed to MRI being made on clinical grounds. An epileptogenic lesion on neuroimaging was defined as a cortically based abnormality highly likely to be the cause of the seizure. Small-vessel disease on CT or MRI was not considered an epileptogenic lesion. EEGs were performed according to a standard protocol using the 10–20 system and recording for 20–30 minutes. The first EEG after the presenting seizure was analyzed at the time of data entry by 2 of the authors (N.L., J.D.).
Patients were classified on the basis of clinical features, EEG, and CT as having had a generalized-onset seizure, focal-onset seizure, or unclassified seizure (“electroclinical diagnosis”). Generalized-onset seizures were defined by no history of prior neurologic insult, absence of focal clinical features or epileptogenic lesion on CT, and the presence of generalized epileptiform abnormalities on EEG. Focal-onset seizures were defined by the presence of at least one of the following: definite focal clinical features, epileptogenic lesion on CT, or focal epileptiform abnormalities on EEG. Unclassified seizures had no focal clinical features, epileptiform abnormalities on EEG, or epileptogenic lesions on neuroimaging.
Patients were reviewed at the clinic 3 to 9 months after the initial seizure and then contacted at regular intervals by phone to determine whether another seizure had occurred. For those who could not be contacted, the linked interhospital computer system and medical records were used to identify any new seizure presentations.
Study aims, outcomes, and statistical analyses
We analyzed the database to answer the following questions: 1) What is the spectrum of neuroimaging findings in an unselected hospital-based group of patients with first-ever unprovoked seizure? 2) How often are epileptogenic lesions identified by MRI when CT is normal? 3) What features predict an epileptogenic lesion when CT is normal? 4) What is the contribution of MRI to the electroclinical diagnosis? and 5) Do neuroimaging findings predict seizure recurrence, including when CT is normal but MRI shows an epileptogenic lesion? Time to seizure recurrence was analyzed with Kaplan-Meier curves and log-rank statistics. Potential risk factors for seizure recurrence were examined using Cox proportional hazards models. Variables significant at the 10% level in preliminary univariate analyses were included in the multivariate Cox proportional hazards regression models. Stepwise logistic regression was performed for analysis of factors that predicted an abnormal MRI when CT was normal. Hazard ratios (HR), odds ratios (OR), and 95% confidence intervals (CIs) were calculated from the final models. Results were considered statistically significant at the 5% level.
Standard protocol approvals, registrations, and patient consents
The study was approved by the medical ethics committee at Royal Perth Hospital.
RESULTS
Clinical and neuroimaging findings
Between 2000 and 2011, 1,013 patients were identified with an unprovoked first-ever seizure (63% male, age range 14–91 years, mean 41 years). Most patients (93%) presented with a tonic-clonic seizure. EEG was performed in 97% of patients. CT brain was performed in 954 (94%), 75% within 24 hours of the first seizure. Contrast was given in 49% of patients. A total of 674 (67%) patients had an MRI brain. A total of 61% had both CT and MRI, 6% had only MRI, and 33% had only CT. Overall, 295 patients (29%) had an epileptogenic lesion on neuroimaging, most commonly stroke, post-traumatic, or neoplastic lesions (table). No patient had an epileptogenic lesion only detected by CT; of 478 patients with a normal CT and subsequent MRI, 57 (12%) were identified to have an epileptogenic lesion on MRI (table). The pathologies most likely to be evident on MRI but not evident on CT were mesial temporal sclerosis, malformations of cortical development, and cavernomas.
Table Epileptogenic lesions identified on any imaging and on MRI when CT was normal
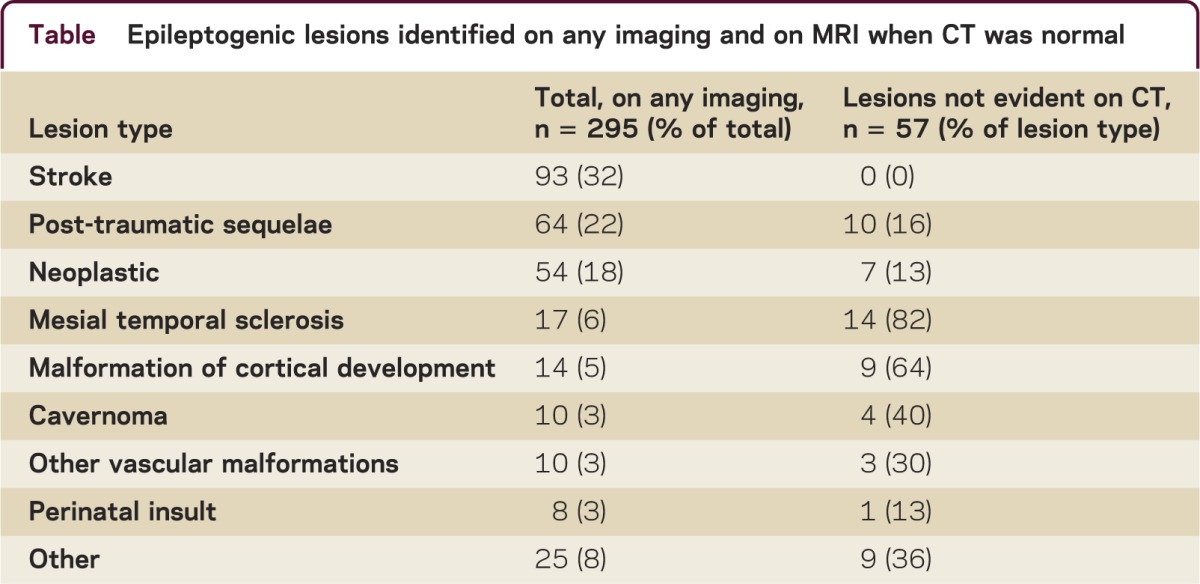
Seven patients (1.5%) with normal CT had a tumor identified on a subsequent MRI. All these patients had a CT on the day of their first seizure, but contrast was given in only one. Five patients had gliomas (2 high-grade), one a ganglioglioma, and one a pleomorphic xanthoastrocytoma. The tumor was in the temporal region in 4 patients. Focal EEG abnormalities were present in 3 of these patients. Seizure recurrence occurred in all 7 patients, within 90 days in 6 patients (all but the patient with the pleomorphic xanthoastrocytoma), and within 30 days in 3 of the 5 patients with gliomas. Early seizure recurrence (within 3 months) predicted patients with tumors not evident on CT (OR 14.3; 95% CI 1.7–120, p = 0.01).
Predictors of epileptogenic lesion on any imaging
Independent predictors of an epileptogenic lesion on any imaging were as follows: history of prior CNS insult: stroke (OR 37.2; 95% CI 14.2–97.0, p < 0.0001), head injury (OR 6.8; 95% CI 4.1–11.3, p < 0.0001), intracranial infection (OR 6.8; 95% CI 1.9–23.6, p = 0.003), birth injury (OR 3.8; 95% CI 1.2–12.0, p = 0.02); focal slowing or epileptiform abnormalities on EEG (OR 5.5; 95% CI 3.8–7.9, p < 0.0001); and simple partial seizures (OR 7.1; 95% CI 2.7–18.6, p < 0.0001).
When CT was normal, the strongest predictor of an epileptogenic lesion on MRI was the presence of focal slowing or epileptiform abnormality on EEG (OR 3.7; 95% CI 2.0–6.7, p < 0.0001). Other predictors were a history of head injury (OR 3.9; 95% CI 1.6–9.2, p = 0.002), intracranial infection (OR 21.2; 95% CI 1.7–260, p = 0.02), or birth injury (OR 5.4; 95% CI 1.1–25.0, p = 0.03). If CT was normal and EEG showed generalized epileptiform abnormalities, then MRI was performed; this occurred in 40 patients, and showed an epileptogenic lesion in only one patient.
Electroclinical diagnosis
Based on clinical data, EEG data, and CT findings, the electroclinical diagnosis was defined as focal in 479 patients (28 based on CT lesion alone), generalized in 89 patients, and unclassified in 445 patients. Of 282 patients with an unclassified tonic-clonic seizure who proceeded to MRI scan, 23 (8.1%) could be reclassified as focal based on the identification of an epileptogenic lesion not seen on CT.
Follow-up and seizure recurrence
Follow-up data of at least 1 year were available in 94% of patients. Survival analysis showed a cumulative likelihood of seizure recurrence at 1 year of 49% (95% CI 46%–52%) and at 2 years of 55% (95% CI 52%–58%). The median time to seizure recurrence was 100 days (range 1–3,291 days). Cox proportional hazards modeling found that independent predictors of seizure recurrence were epileptogenic lesion on imaging (HR 1.39; 95% CI 1.17–1.66, p < 0.001), first seizure from sleep (HR 1.26; 95% CI 1.04–1.51, p = 0.01), epileptiform abnormality on EEG (HR 1.36; 95% CI 1.11–1.67, p = 0.003), and focal seizures (HR 1.61; 95% CI 1.18–2.22, p = 0.003).
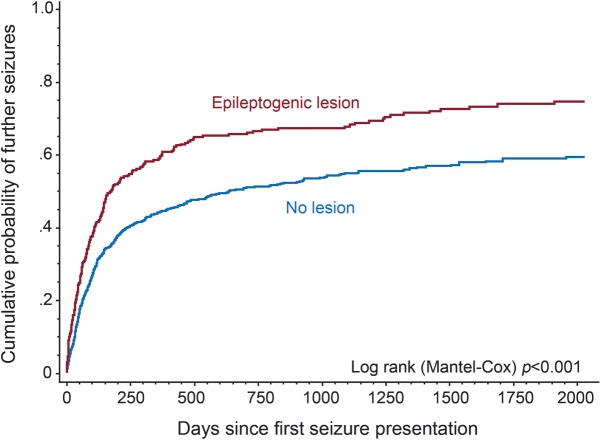
The cumulative likelihood of seizure recurrence (figure 1) at 1 year was 59% (95% CI 54%–65%) for those with an epileptogenic lesion on any imaging, and 44% (95% CI 41%–48%) for those without a lesion (log-rank p < 0.001).
Cumulative probability of second seizure for epileptogenic lesion on any neuroimaging (CT or MRI)
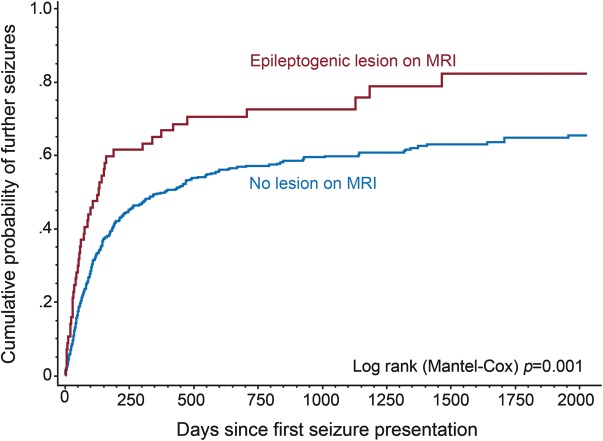
In the 478 patients with normal CT head and in whom MRI was performed, an epileptogenic lesion identified on MRI also predicted seizure recurrence (figure 2): the cumulative likelihood of seizure recurrence at 1 year was 67% (95% CI 54%–79%) for those with an epileptogenic lesion only seen on MRI, and 50% (95% CI 45%–55%) for those without a lesion (log-rank p = 0.001).
Cumulative probability of second seizure for epileptogenic lesion found on MRI when prior CT was normal
DISCUSSION
MRI is clearly superior to CT in detection of epileptogenic abnormalities in patients with first-ever unprovoked seizure, in particular mesial temporal sclerosis and malformations of cortical development. MRI detected a lesion not evident on CT in 1 of 8 patients, the undetected lesion being a tumor in 1 of 67 patients. A history of prior CNS insult or focal slowing or epileptiform abnormalities on EEG predicted an epileptogenic lesion identified by MRI when CT was normal. Patients with tumors missed by CT characteristically had an early seizure recurrence. Our findings are similar to a previous publication where 6% of patients with first seizure presentation were identified to have relevant lesions on MRI, although a large proportion of patients in that study had prior seizures and patients with an “obvious” cause were excluded.5 MRI provided additional information regarding the electroclinical diagnosis, allowing reclassification of 8% of patients after normal CT. We have also confirmed that an epileptogenic lesion identified on imaging predicts seizure recurrence, including when a lesion is only evident on MRI.
Our data have been prospectively collected in a large well-defined group of patients with comprehensive follow-up, but this is a pragmatic, predominantly hospital-based observational study with several limitations. MRI was performed in 67% of patients, when considered necessary on clinical grounds, and this may have biased the results toward finding a lesion on MRI, and some patients had MRI after seizure recurrence. Although scanner quality and protocols evolved over the 10-year study period, the annual proportion of patients in whom an epileptogenic lesion was detected by CT or MRI did not significantly change.
Our findings suggest that if MRI is readily available then it can replace CT in most patients with first-ever seizure and provide additional diagnostic and prognostic information. However, while normal CT in patients with first-ever unprovoked seizure is less sensitive than MRI, CT is unlikely to miss a substantial lesion and patients with tumors missed by CT characteristically had an early seizure recurrence. If MRI is not readily available, initial imaging with CT is reasonable; if CT is normal, then consider MRI if seizures recur or if focal EEG abnormalities are present. This approach to utilization of imaging after first-ever seizure in adults may be applicable in a resource-limited environment, relevant to much of the world's population.
STUDY FUNDING
Funding for the establishment and maintenance of the first-seizure database in the form of a grant to cover the costs of a research assistant was received from the Medical Research Foundation of Royal Perth Hospital and UCB-Pharma.
DISCLOSURES
K. Ho reports no disclosures. N. Lawn has received funding for travel from UCB Pharma and research support from UCB Pharma and from the Royal Perth Hospital Medical Research Foundation. M. Bynevelt and J. Lee report no disclosures. J. Dunne has received funding for travel from UCB Pharma and from several nonprofit organizations. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a78f25.
Correspondence to: nicholaslawn@yahoo.com
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a78f25.
Footnotes
Correspondence to: nicholaslawn@yahoo.com
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a78f25.
REFERENCES
- 1.Harden CL, Huff JS, Schwartz TH. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review) Neurology. 2007;69:1772–1781. doi: 10.1212/01.wnl.0000285083.25882.0e. [DOI] [PubMed] [Google Scholar]
- 2.Krumholz A, Wiebe S, Gronseth G. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review) Neurology. 2007;69:1996–2007. doi: 10.1212/01.wnl.0000285084.93652.43. [DOI] [PubMed] [Google Scholar]
- 3.Bernal B, Altman NR. Evidence-based medicine: neuroimaging of seizures. Neuroimaging Clin N Am. 2003;13:211–224. doi: 10.1016/s1052-5149(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 4.So EL. Role of neuroimaging in the management of seizure disorders. Mayo Clin Proc. 2002;77:1251–1264. doi: 10.4065/77.11.1251. [DOI] [PubMed] [Google Scholar]
- 5.King MA, Newton MR, Jackson GD. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998;352:1007–1111. doi: 10.1016/S0140-6736(98)03543-0. [DOI] [PubMed] [Google Scholar]
- 6.Kho LK, Lawn ND, Dunne JW, Linto J. First seizure presentation: do multiple seizures within 24 hours predict recurrence? Neurology. 2006;67:1047–1049. doi: 10.1212/01.wnl.0000237555.12146.66. [DOI] [PubMed] [Google Scholar]
- 7.Commission on Epidemiology and Prognosis, International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia 1993;34:592–596. [DOI] [PubMed]