Figure 5.
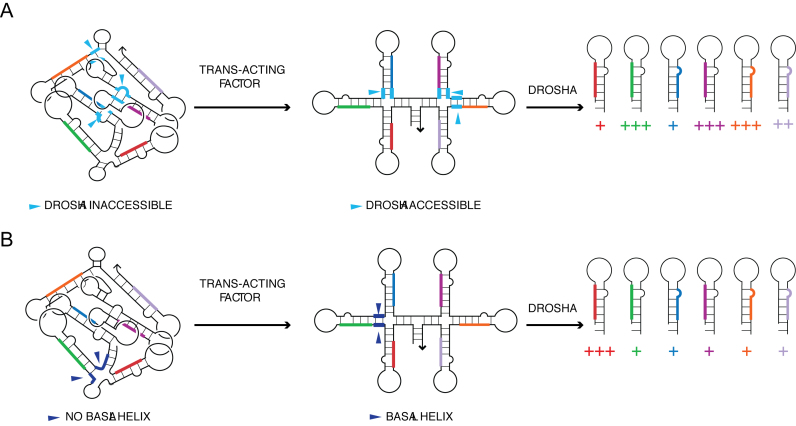
Modes by which tertiary structure of pri-miRNA influences differential processing of constituent miRNAs. (A) Pri-miRNA domains can have their basal helices (cyan segments) and/or Drosha cut sites buried, making them inaccessible to Drosha/DGCR8, impeding their microprocessing. Binding of a trans-acting factor is needed to unfold the tertiary structure to provide accessibility to Drosha. (B) In the fully folded structure, pri-miR-92a does not have a basal helix (dark blue segments). The binding of a trans-acting factor is necessary to generate a basal helix and make the primary microRNA domain a substrate for Drosha.