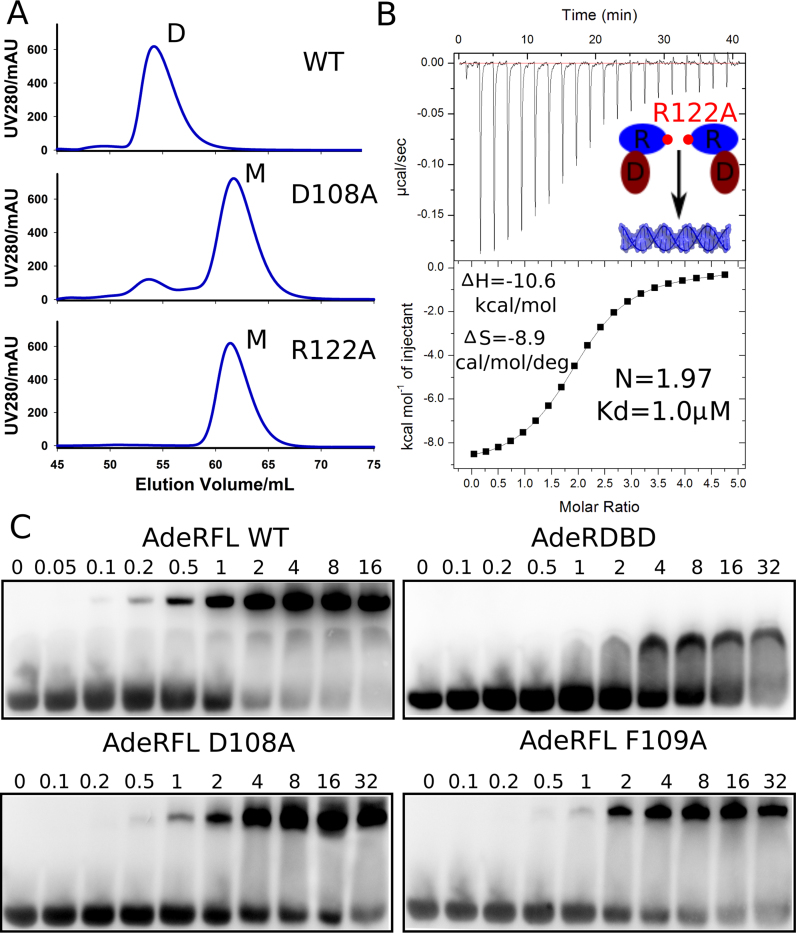
Figure 3.
Mutagenesis studies of AdeR receiver domain. (A) Size-exclusion chromatography analysis shows that the residues D108A and R122A could disrupt the full length dimerization of AdeR. (B) Isothermal titration calorimetry experiment indicates the thermodynamics of AdeR full length R122A mutation exhibits a similar profile as the AdeR DNA binding domain alone. (C) Electrophoretic mobility shift assay validates that D108A and F109A interact with the intercistronic DNA similar to the AdeR DNA binding domain alone in a much lower affinity compared to wildtype AdeR full length.