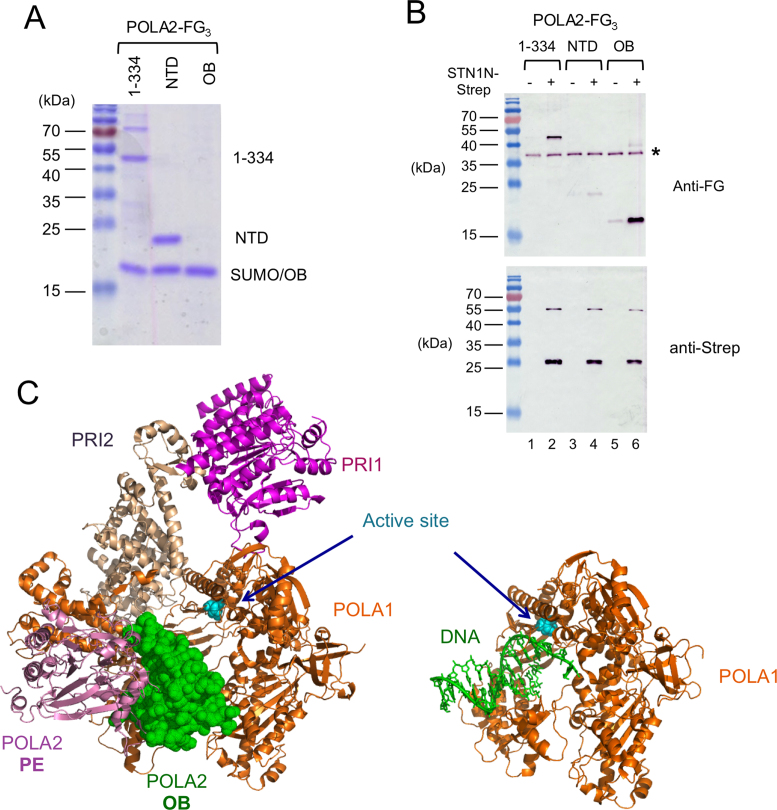
Figure 6.
OB–OB interaction between STN1 and POLA2, and the steric clash between DNA and POLA2 OB fold domain near the Pol α active site. (A) Truncated POLA2 variants were expressed as His-SUMO-POLA2-FG3 fusions, purified by Ni-NTA and then subjected to ULP1 cleavage. The resulting preparations that were used as inputs for STN1N-Strep pull down assays were subjected to SDS-PAGE and Coomassie staining. About 1/30 of the input samples were analyzed. Note that the mobility of OB domain is identical to the SUMO fragment. (B) POLA2 fragments were subjected to pull down by STN1N, and the samples eluted from Strep-Tactin beads were subjected to western blot analysis using both anti-FLAG and anti-Strep antibodies. A contaminant that cross-reacts with the FLAG antibody is indicated by *. (C) The crystal structure of the human primase-pol α complex without substrates (PDB ID: 5EXR) is shown on the left and that of a polymerase–DNA complex (PDB ID: 5IUD) shown on the right. The different polypeptides and the DNA are displayed in different colors as matched by the text labels. The active site aspartates of the DNA polymerase are shown in cyan and in sphere representations. The POLA1 polypeptide in the two structures are displayed in the same orientation to illustrate the point that the OB fold of POLA2 in the apo complex occupies the same position as the DNA substrate in the polymerization complex.