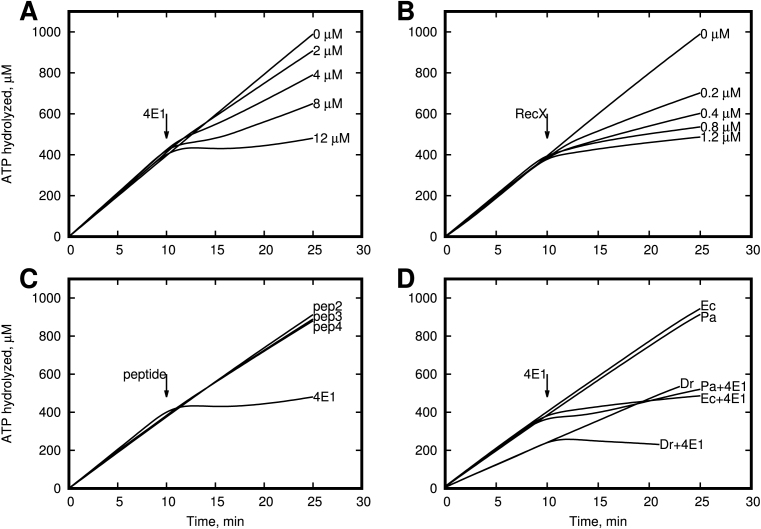
Figure 3.
Effect of α-helical peptide 4E1 on ATP activity of the RecA protein in vitro. (A) 4E1 peptide inhibits RecA ATPase activity on poly-(dT). In the absence of any inhibitors the amount of hydrolyzed ATP increases proportionally to reaction time. (B) RecX protein inhibits RecA ATPase activity on poly-(dT). In the absence of any inhibitors the amount of hydrolyzed ATP increases proportionally to reaction time. (C) Peptides Escherichia coli RecA ATPase activity on poly-(dT). In the absence of peptide 4E1 (12 μM) the amount of hydrolyzed ATP increases proportionally to reaction time. (D) RecA protein from E. coli, Deinococcus radiodurans and Pseudomonas aeruginosa ATPase activity on poly-(dT) inhibited by peptide 4E1 (12 μM).