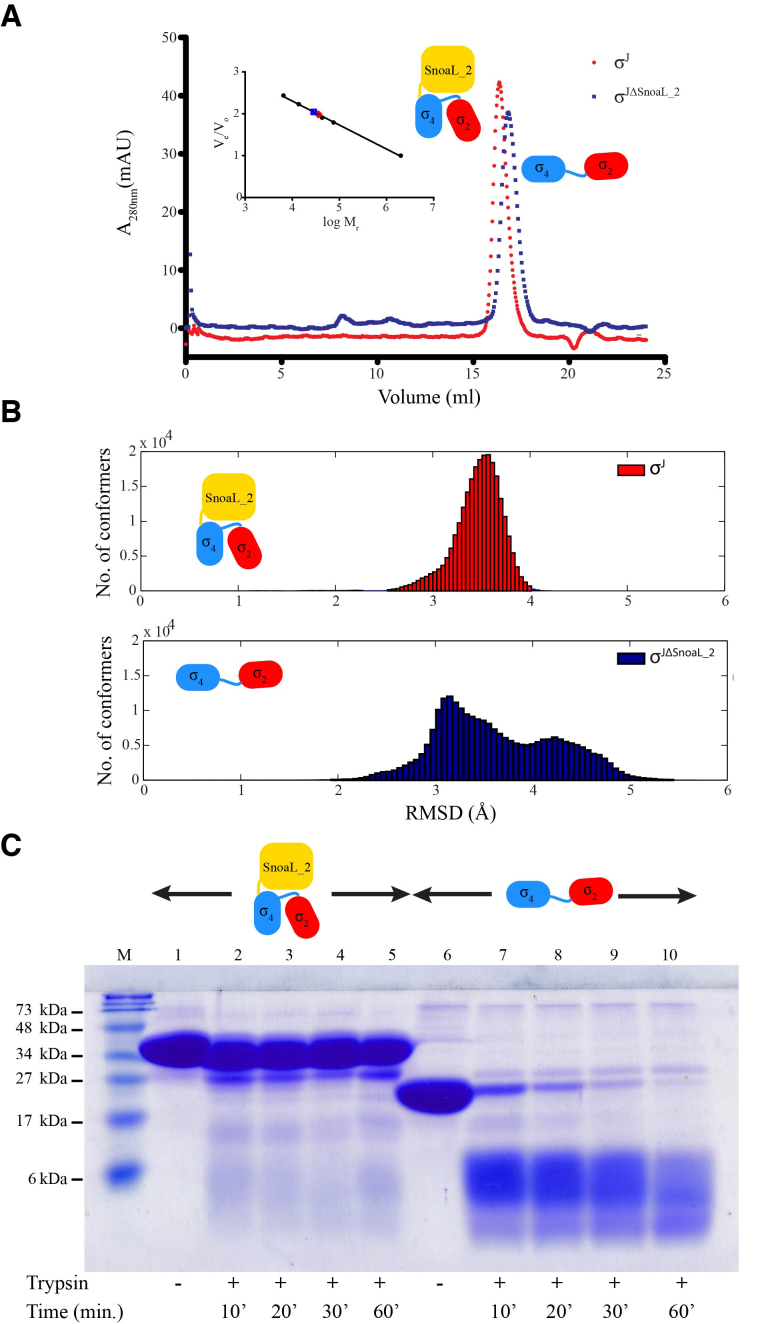
Figure 4.
The SnoaL_2 domain contributes to a compact σJ conformation. (A) Comparison of analytical size exclusion chromatogram of σJ and σJΔSnoaL_2. The apparent molar mass (28.5 kDa) of the σJΔSnoaL_2 protein is higher than expected (19.1 kDa) for a monomer. (B) Molecular dynamics (MD) simulations provide a basis for a comparison between the conformations sampled by σJ and σJΔSnoaL_2. The considerable conformational heterogeneity noted in the MD simulations for σJΔSnoaL_2 is consistent with the hypothesis that the SnoaL_2 domain constraints σJ to a compact structure. (C) A comparison between the proteolytic susceptibility of σJ and σJΔSnoaL_2 (using trypsin) revealed that σJΔSnoaL_2 is significantly more proteolytically labile when compared to full length σJ.