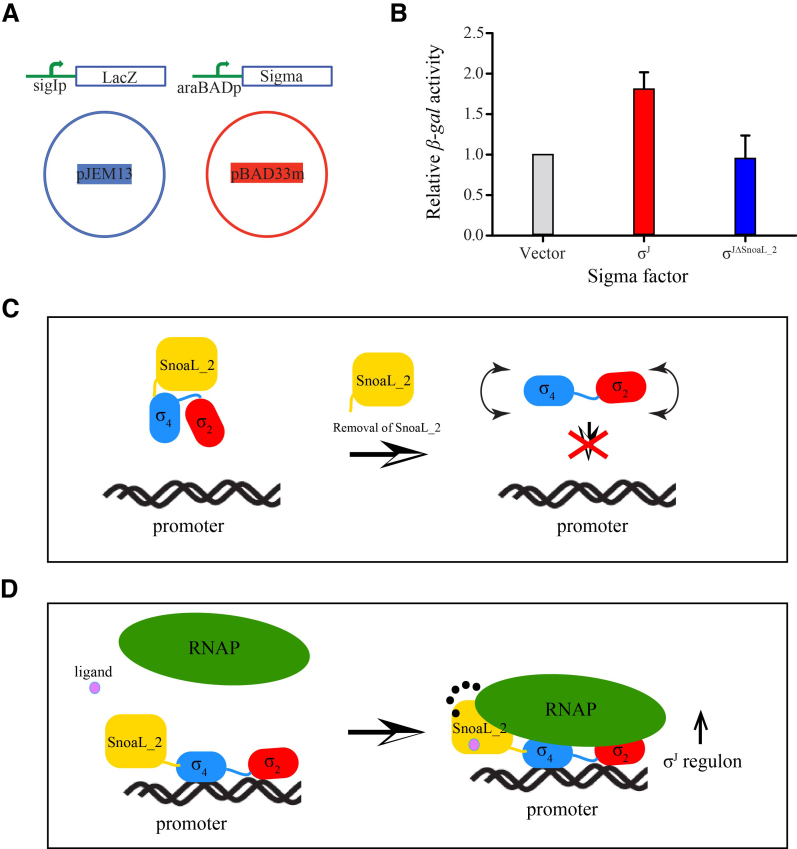
Figure 5.
The SnoaL_2 domain modulates σJ activity. (A) A schematic representation of the β-galactosidase assay to evaluate σJ activity. The target plasmid contained a sigI-promoter fused to a lacZ gene cloned in the pJEM13 vector (56). The donor plasmid contained either σJ or σJΔSnoaL_2 cloned in the pBAD33m vector. The pBAD33m vector served as a control in this experiment. (B) β-galactosidase activity was significantly reduced upon deleting the SnoaL_2 domain (σJΔSnoaL_2) when compared to the full-length σ factor. The SnoaL_2 domain is thus unlikely to play the role of a σ factor antagonist (anti-σ factor). (C and D) A mechanistic model for σJ activity. The influence of the Snoal_2 domain on DNA binding and transcription initiation suggests that the C-terminal domain is an essential part of σJ (C). The SnoaL_2 domain tethers σJ2 and σJ4 in an orientation compatible with promoter DNA binding and potentially influences σJ–RNAP interaction (16). These interactions could be modulated by the binding of small molecules to the SnoaL_2 domain (D). The SnoaL_2 domain can thus modulate σJ activity by inducing conformational changes that regulate promoter DNA or RNAP binding.