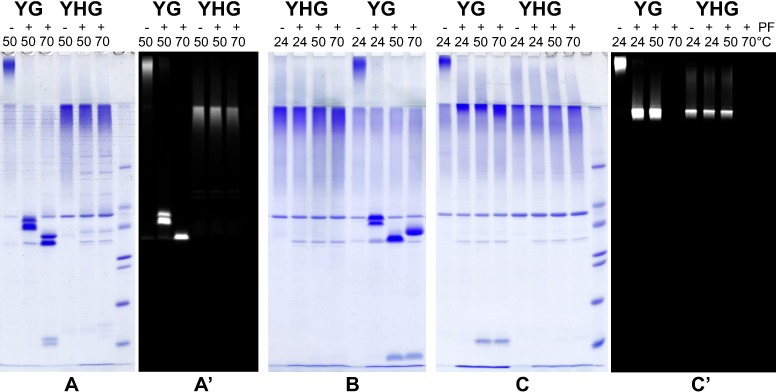
Fig 13. Comparison of the properties of secreted Ywp1-Gfp and Ywp1-6HA-Gfp.
Strain YHY was transfected with a cassette that inserted GFP into either its YWP1 allele or its YWP1-6HA-YWP1 allele, generating comparator strains that secreted either Ywp1-Gfp (“YG”) or Ywp1-6HA-Gfp (“YHG”), as indicated. The Gfp was fused to either aa 165 (A,B) or to aa 520 (C) of Ywp1. Cultures were grown for 45–47 hr in 30°C BMM13 that started with 0.3 mM phosphate. After alkalinization of the culture supernatants to pH 8, secreted proteins were concentrated by centrifugal ultrafiltration. Aliquots were heated to the indicated temperatures (24, 50 or 70°C) before (A) or after (B,C) digestion (or mock digestion) with PNGase F (“PF”, which removes the N-glycan from Ywp1 aa 115). Digested (+) and undigested (-) samples were mixed with excess SDS (and DTT for A and C) and incubated at 24°C (A) or at the indicated temperatures (B and C) prior to SDS-PAGE, which was followed by laser scanning for Gfp fluorescence (A’,C’) and subsequent staining for protein with Coomassie Blue R-250 (A,B,C). In the presence of excess SDS (B,C), heating to 70°C denatured the Gfp (and rendered it nonfluorescent); in the absence of excess SDS (A), denaturation by 70°C was much less efficient. Markers in A and C are as in S2 Fig. The Ywp1 propeptide bands at the bottom of the gels exhibit slight degradation in an older sample (A) and greater mobility without disulfide reduction (B), as noted previously [21, 22].