Fig. 2.
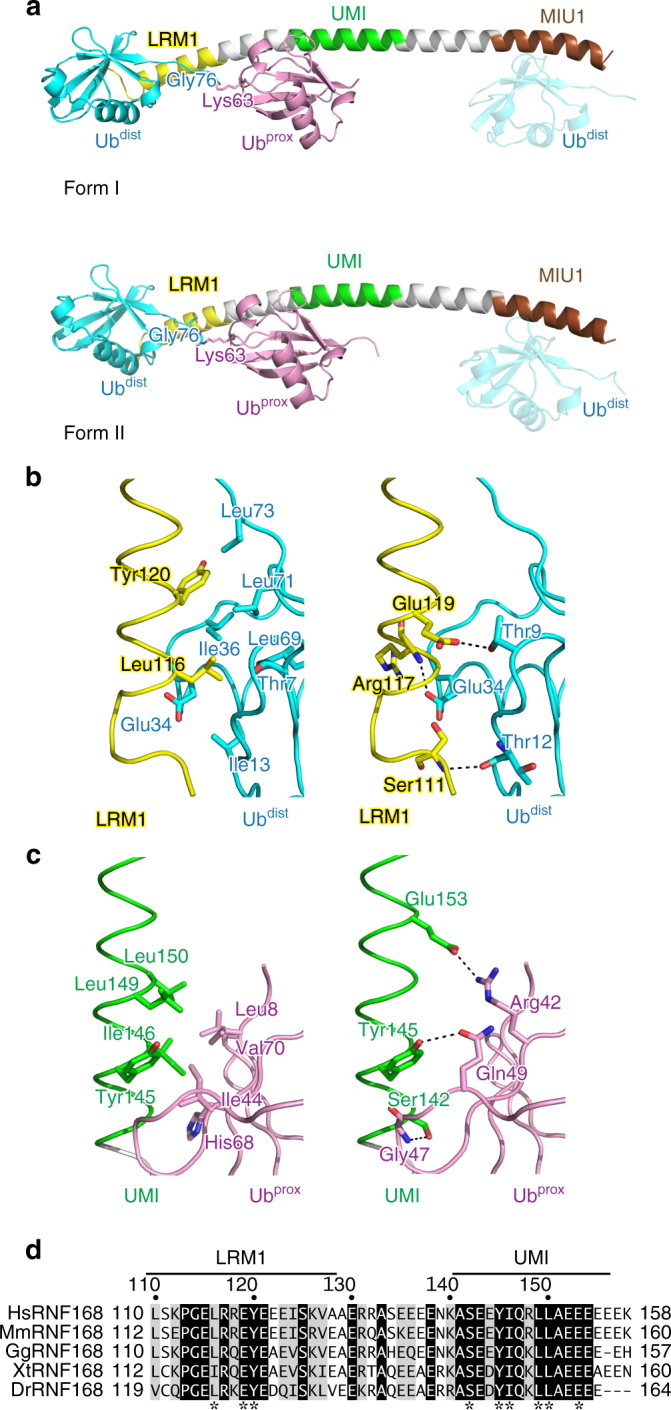
Structure of the complex between RNF168 UDM1 and K63-Ub2. a Overall structure of the complex. The coloring scheme of UDM1 is the same as in Fig. 1. Ubdist and Ubprox of K63-Ub2 are colored cyan and pink, respectively. The linkage between Gly76 of Ubdist and Lys63 of Ubprox is shown. The MIU1-bound Ubdist from the adjacent complex in the crystal is also shown as a translucent model. b Hydrophobic (left) and hydrogen-bonding (right) interactions between RNF168 LRM1 and Ubdist. The coloring scheme is the same as in a, c Hydrophobic (left) and hydrogen-bonding (right) interactions between RNF168 UMI and Ubprox. The coloring scheme is the same as in a, d Sequence alignment of LRM1–UMI in human (Hs), mouse (Mm), chicken (Gg), xenopus (Xt), and zebrafish (Dr) RNF168 proteins56]. Fully conserved residues are colored white with black background, whereas residues with similar properties (scoring >0.5 in the Gonnet matrix57) are marked with gray backgrounds. Asterisks represent the residues whose side chains interact with K63 chains
