Figure 6.
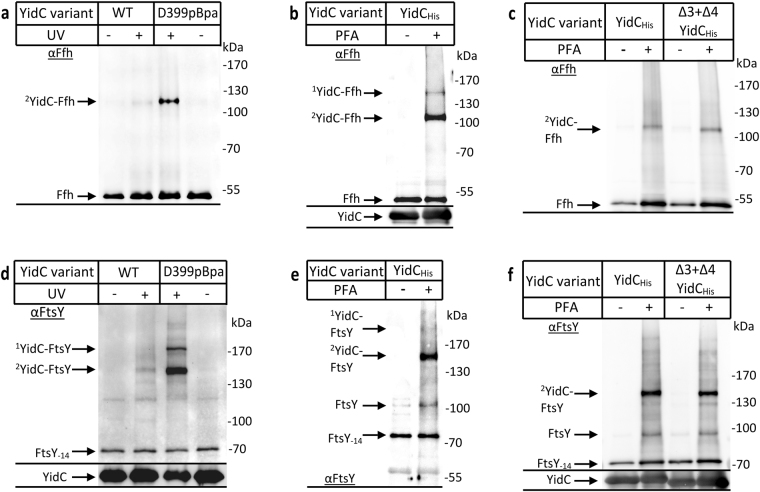
The cytosolic loop 2 of YidC constitutes a binding site for SRP and its receptor FtsY. (a) In vivo photo-cross-linking was performed in the single-expression system using BL21 cells expressing yidC with pBpa at position 399. Subsequently YidC was purified via metal affinity chromatography and analysed on western blot with antibodies directed against Ffh, the protein subunit of the E. coli SRP. The 110 kDa YidC-Ffh cross-link is indicated. Note, a very weak, apparently also UV-dependent band was recognized by α-Ffh antibodies in the wild type. (b) BL21 cells expressing YidC were treated in vivo with p-formaldehyde (PFA) or buffer as a control. Subsequently, YidC was purified together with its cross-linking partners by metal affinity chromatography and analysed on western blot with α-Ffh antibodies. The 1YidC-Ffh species likely corresponds to a YidC-Ffh-4.5SRNA cross-link product and the 2YidC-Ffh species to the YidC-Ffh cross-link. (c) As in (b), but with cells expressing either wild type YidC or the YidC(Δ3 + Δ4) variant, which lacks most of the C1-loop. (d) The same material shown in (a) was decorated with α-FtsY antibodies, which revealed two cross-link products migrating at about 150 kDa and 180 kDa. FtsY-14 corresponds to the proteolytic cleavage product of FtsY, which is frequently observed. (e,f) The same material as in (b,c) was decorated with α-FtsY antibodies. Uncropped images are displayed in Supplementary Figure S4.