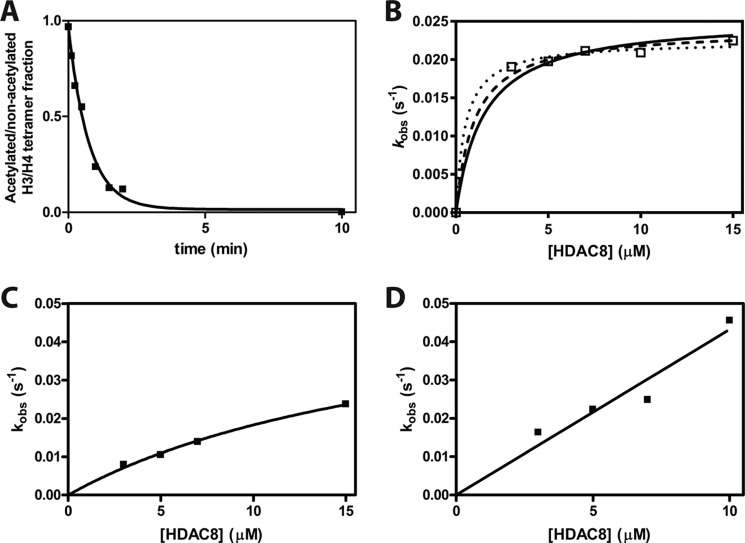
Figure 2.
Single turnover deacetylation of singly-acetylated H3/H4 tetramers. A, sample data from a deacetylation reaction (7 μm HDAC8 and 0.5 μm H3K9ac/H4 tetramer (1 μm acetyl-lysine)) measured using mass spectrometry. The time-dependent decrease in acetylated protein is best described by a single exponential. B, dependence of apparent deacetylation rate constant of H3K9ac/H4 on the concentration of HDAC8. The kobs average of 0.021 ± 0.001 s−1 shows little dependence on the [HDAC8]. Three separate hyperbolic fits are shown that bracket potential K½ values: K½ = 0.5 μm (dotted line); K½ = 1.0 μm (dashed line); and K½ = 1.5 μm (solid line). These fits demonstrate that the K½ is <1.5 μm and kmax/K½ is >17,000 m−1 s−1. The data points are from multiple measurements in a single reaction at each HDAC8 concentration. C, dependence of the deacetylation rate constant for H3K14ac/H4 on the concentration of HDAC8. The data points are from multiple measurements in a single reaction at each HDAC8 concentration. A hyperbolic fit indicates that the kmax/K½ is 2,500 ± 70 m−1 s−1 with an estimated value for kmax of 0.06 s−1. D, dependence of the deacetylation rate constant for H3K56ac/H4 on the concentration of HDAC8. The data points are from multiple measurements in a single reaction at each HDAC8 concentration. A linear fit indicates that the kmax/K½ is 4,000 ± 600 m−1 s−1.