Abstract
Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
To the editor:
Today, the large majority of patients with Hodgkin lymphoma are cured.1-6 However, treatment of patients with advanced-stage disease with optimal chemotherapy, such as doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), fails to cure as many as 15% to 25% of patients, necessitating secondary intensified treatment, which incurs major toxicity and is only successful in ∼50% of patients.1,7,8 Genuine improvement in these outcomes will require identification of novel effective agents that can be integrated with standard chemotherapy during primary treatment. One candidate agent is brentuximab vedotin (ADCETRIS, Seattle Genetics, Inc), an antibody drug conjugate that employs a protease cleavable covalent linker to combine a chimeric monoclonal antibody directed against the CD30 antigen found universally on the surface of Hodgkin Reed-Sternberg cells with monomethyl auristatin E, a microtubule disrupting agent. Brentuximab vedotin has substantial effectiveness (complete response rate 34%; overall response rate 75%9) against otherwise treatment-resistant classical Hodgkin lymphoma and confers only modest toxicity, primarily confined to reversible neuropathy, suggesting that it may be safely combined with standard primary chemotherapy. We previously conducted a phase 1 clinical trial with an expansion cohort testing the safety and effectiveness of the addition of brentuximab vedotin to ABVD or the same combination with omission of bleomycin.10 The addition of brentuximab vedotin was associated with excellent outcomes, including complete response in 45 of 47 assessable patients (95%). The addition of brentuximab vedotin was well tolerated, permitting full doses of 1.2 mg/kg every 2 weeks to be delivered without compromising the dosing of the other therapeutic chemotherapy agents; however, excessive pulmonary toxicity emerged when brentuximab vedotin was added to ABVD, indicating that brentuximab vedotin and bleomycin cannot be safely combined. At the conclusion of the original trial, we initiated a long-term follow-up study to determine the ultimate impact of the addition of brentuximab vedotin to ABVD or doxorubicin, vinblastine, and dacarbazine (AVD).
In brief, 51 patients with previously untreated advanced-stage (stage IIA bulky, n = 3; IIB, n = 8; IIIA, n = 8; IVA, n = 9; IVB, n = 23) classical Hodgkin lymphoma were treated with ABVD (n = 25) or AVD (n = 26) plus brentuximab vedotin.10 Patients’ initial characteristics are summarized in Table 1. Brentuximab vedotin was given IV every 2 weeks on the same day as the ABVD or AVD, and the dose was escalated from 0.6 mg/kg to 1.2 mg/kg in planned cohorts (0.6 mg/kg, n = 6; 0.9 mg/kg, n = 13; 1.2 mg/kg, n = 6). All 26 patients treated with AVD started their brentuximab vedotin at the full planned dose of 1.2 mg/kg every 2 weeks (n = 26). Additional details, including use of supportive medications such as neutrophil growth factors, are fully described in the original publication about this trial.10 Radiation, permitted at the treating physician’s discretion, was delivered to 4 patients (8%) at the conclusion of primary chemotherapy. Three of these 4 patients had bulky mediastinal tumors, and all 4 patients were treated with AVD plus brentuximab vedotin. None experienced pulmonary toxicity.
Table 1.
Patient characteristics
| ABVD + brentuximab vedotin | AVD + brentuximab vedotin | |
|---|---|---|
| n | 25 | 26 |
| Age, y, median (range) | 35 (19-59) | 33 (18-58) |
| Male sex, n (%) | 20 (80) | 17 (65) |
| Performance status,* n (%) | ||
| 0 | 13 (52) | 11 (42) |
| 1 | 12 (48) | 15 (58) |
| Stage, n (%) | ||
| IIA bulky† | 0 | 3 (12) |
| IIB | 4 (16) | 4 (15) |
| IIIA | 5 (20) | 3 (12) |
| IVA | 4 (16) | 5 (19) |
| IVB | 12 (48) | 11 (42) |
| International Prognostic Score, n (%) | ||
| 0-3 | 5 (20) | 12 (46) |
| 4-7 | 20 (80) | 14 (54) |
Eastern Cooperative Oncology Group scale.
Bulky = any mass ≥10 cm.
After completion of the original trial, patients were enrolled in this long-term follow-up study. Periodically, each patient’s current vital and relapse status was determined and, if relapse had occurred, the date and planned treatment of relapse were recorded, after which only vital status and presence or absence of lymphoma were subsequently ascertained. Survival estimates were calculated using the method of Kaplan and Meier, and graphical plots were displayed using GraphPad Prism 7.0 software. Failure-free survival (FFS) was the interval from diagnosis to death from any cause, disease progression after initiation of primary treatment, or date of last follow-up. Overall survival (OS) was the interval from diagnosis to death from any cause or date of last follow-up. This study was approved by the respective institutional research ethics boards of each of the participating centers. Seattle Genetics, Inc provided funding to support the conduct of the study and was provided a copy of this manuscript in advance of publication but did not otherwise participate in its execution or interpretation.
Two patients died because of pulmonary toxicity during treatment with ABVD plus brentuximab vedotin, and 1 patient initially treated with ABVD plus brentuximab withdrew consent after 1 cycle of treatment, leaving 48 patients available for the long-term follow-up study. Their median follow-up has now reached 66 months (range 45 to 84 months). Three patients were lost to follow-up at 10, 22, and 28 months while still in first complete remission.
Three of 22 patients given ABVD plus brentuximab vedotin relapsed at 2, 15, and 16 months and 2 of 26 given AVD plus brentuximab vedotin relapsed at 1 and 15 months, respectively. All 5 patients who relapsed underwent high-dose chemotherapy and autologous hematopoietic stem cell transplantation. Four remain in second complete remission 21, 33, 52, and 55 months from the time of transplantation, and 1 has relapsed again and is receiving single-agent chemotherapy. Of the 43 patients who have remained free of relapse, 41 have been followed for longer than the longest time to relapse (16 months) and 39 have been followed for longer than 45 months since diagnosis. Among the 48 patients enrolled in this long-term study, there have been no deaths, although 1 patient remains alive with lymphoma and is receiving palliative chemotherapy.
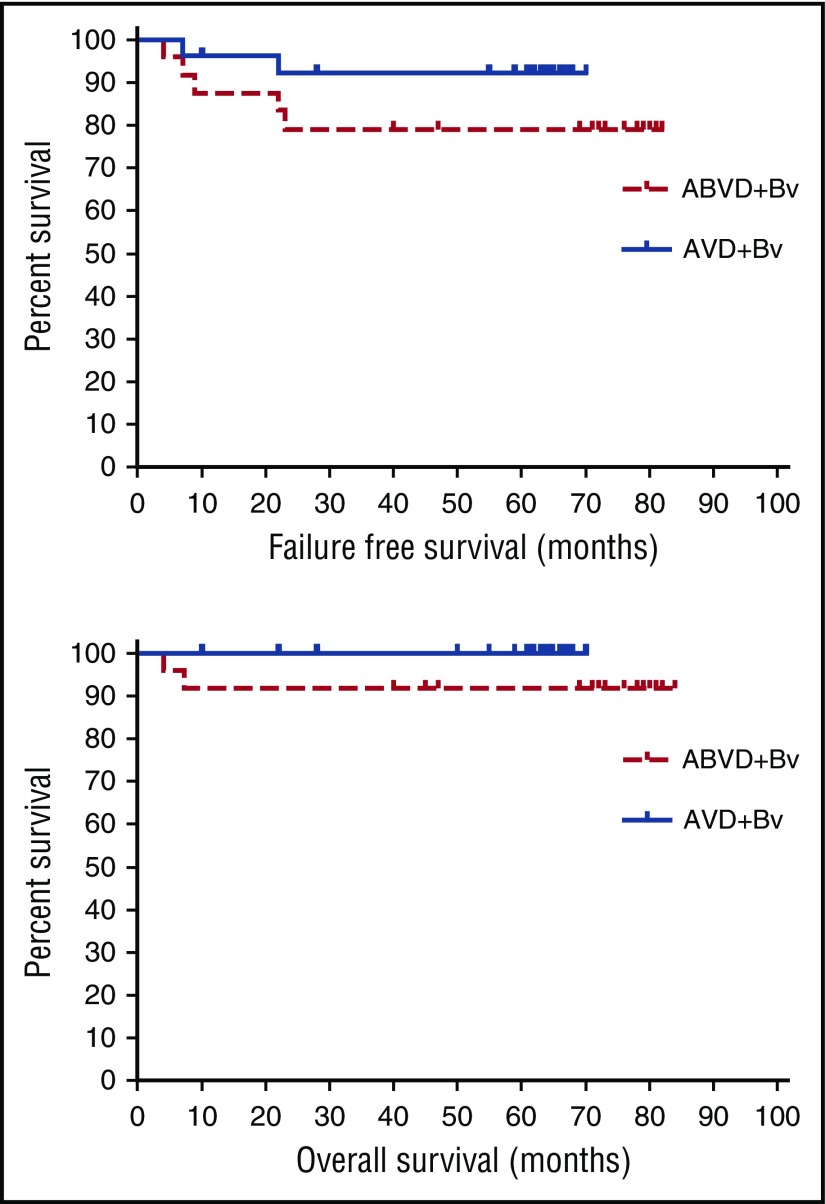
The 26 patients who received AVD plus brentuximab vedotin are of particular interest because they received treatment matching the experimental arm of the ECHELON-1 trial comparing standard ABVD with AVD plus brentuximab vedotin for patients with stage III or IV classical Hodgkin lymphoma. Among these patients, there have only been 2 relapses, and all 26 remain alive and free of lymphoma, 24 in first and 2 in second remission. The 5-year FFS and OS were 79% and 92% after ABVD plus brentuximab vedotin and 92% and 100% after AVD plus brentuximab vedotin, respectively (Figure 1). Of particular note is the maturity of the follow-up. All patients on the AVD plus brentuximab vedotin arm have now been followed for longer than the maximum time to primary treatment failure with the exception of 2 patients lost to follow-up while in first complete remission 10 and 22 months after diagnosis.
Figure 1.
FFS and OS for patients with advanced-stage classical Hodgkin lymphoma after primary treatment with ABVD + brentuximab vedotin (ABVD+Bv) or AVD + brentuximab vedotin (AVD+Bv).
Several important observations emerged from this study. During the phase 1 dose escalation, it became clear that brentuximab vedotin cannot be safely combined with bleomycin because of excessive pulmonary toxicity. The expansion AVD plus brentuximab vedotin part of the trial demonstrated that brentuximab vedotin can be safely and tolerably combined with AVD at full therapeutic doses.10 More importantly, this combination resulted in excellent and durable control of advanced-stage classical Hodgkin lymphoma with a very high complete response rate (96%) and estimated 5-year FFS and OS of 92% and 100%, respectively, which compare favorably with recently reported results using ABVD for similar patients with advanced-stage Hodgkin lymphoma (complete response 73%; 5-year FFS 74%; 5-year OS 88%11) The potential for the combination of AVD and brentuximab vedotin to prove superior to standard ABVD for the treatment of classical Hodgkin lymphoma is being examined in the large international prospective randomized ECHELON-1 trial, which has completed enrollment and should be available for initial analysis in the near future.
Authorship
Acknowledgments: The authors thank Elena Moon for data management at the British Columbia Cancer Agency, the patients who participated in this study, and the many physicians who supported this trial by referral of patients.
Contribution: All authors participated in the study’s conception, data gathering, analysis, interpretation, and manuscript drafting.
Conflict-of-interest disclosure: J.M.C.’s institution receives support for research from F Hoffmann-La Roche; Bristol-Myers Squibb; Takeda Pharmaceutical Company; and Seattle Genetics. S.I.P. receives research support from Seattle Genetics and Teva; serves on advisory boards for Cornerstone Pharmaceuticals and Teva; and serves on a speaker’s bureau for Gilead. S.M.A.’s institution receives support for research from Takeda Pharmaceutical Company and Seattle Genetics. A.Y. receives honoraria for consulting from Merck, Bristol-Myers Squibb, Bayer, Celgene, Incyte, Sanofi, Janssen R&D, Seattle Genetics, and Takeda Millennium; and research funding from Novartis, Johnson and Johnson, Curis, Roche, and Bristol-Myers Squibb. M.F. serves in a consulting role or on an advisory board for Bristol-Myers Squibb and Merck; receives honoraria from Seattle Genetics and Takeda; and receives research funding from Bristol-Myers Squibb, Merck, Takeda, and Seattle Genetics.
Correspondence: Joseph M. Connors, British Columbia Cancer Agency Centre for Lymphoid Cancer, 600 West 10th Ave, Vancouver, BC, Canada V5Z 4E6; e-mail: jconnors@bccancer.bc.ca.
References
- 1.Merli F, Luminari S, Gobbi PG, et al. . Long-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: a study by Fondazione Italiana Linfomi. J Clin Oncol. 2016;34(11):1175-1181. [DOI] [PubMed] [Google Scholar]
- 2.Carde P, Karrasch M, Fortpied C, et al. . Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, International Prognostic Score ≥ 3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 Intergroup Trial. J Clin Oncol. 2016;34(17):2028-2036. [DOI] [PubMed] [Google Scholar]
- 3.Radford J, Illidge T, Counsell N, et al. . Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598-1607. [DOI] [PubMed] [Google Scholar]
- 4.Connors JM. Risk assessment in the management of newly diagnosed classical Hodgkin lymphoma. Blood. 2015;125(11):1693-1702. [DOI] [PubMed] [Google Scholar]
- 5.Press OW, Li H, Schöder H, et al. . US Intergroup Trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016;34(17):2020-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hapgood G, Zheng Y, Sehn LH, et al. . Evaluation of the risk of relapse in classical Hodgkin lymphoma at event-free survival time points and survival comparison with the general population in British Columbia. J Clin Oncol. 2016;34(21):2493-2500. [DOI] [PubMed] [Google Scholar]
- 7.Alinari L, Blum KA. How I treat relapsed classical Hodgkin lymphoma after autologous stem cell transplant. Blood. 2016;127(3):287-295. [DOI] [PubMed] [Google Scholar]
- 8.Gerrie AS, Power MM, Shepherd JD, Savage KJ, Sehn LH, Connors JM. Chemoresistance can be overcome with high-dose chemotherapy and autologous stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Ann Oncol. 2014;25(11):2218-2223. [DOI] [PubMed] [Google Scholar]
- 9.Younes A, Gopal AK, Smith SE, et al. . Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes A, Connors JM, Park SI, et al. . Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348-1356. [DOI] [PubMed] [Google Scholar]
- 11.Gordon LI, Hong F, Fisher RI, et al. . Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]