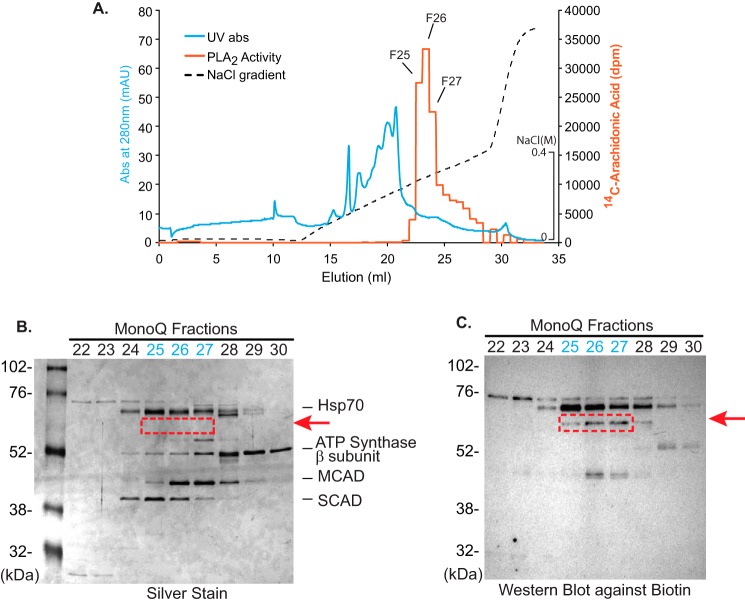
Figure 7.
Purification of the predominant mitochondrial Ca2+-dependent PLA2 in non-failing human myocardial mitochondria through activity-based protein profiling following multiple chromatographic purification steps. To detect the putative serine hydrolase(s) responsible for the observed PLA2 activity, mitochondria isolated from non-failing human myocardium were resuspended in highly basic buffer (20 mm Na2CO3 (pH 11.4)), and the soluble fraction was collected by ultracentrifugation at 100,000 × g. Next, the mitochondrial extract was applied to sequential DEAE and chromatofocusing Mono P columns as described under “Experimental procedures” (Fig. S4). Fractions with phospholipase A2 activity (identified using [14C]PAPC as substrate) were collected at each chromatographic step for high-resolution separation utilizing a Mono Q FPLC column. A, elution profile of the identified PLA2 activity following Mono Q chromatography as monitored by UV absorbance (280 nm) and release of [14C]arachidonic acid from [14C]PAPC. Active fractions from the Mono Q FPLC column were resolved by SDS-PAGE and visualized by silver staining (B) or were incubated with desthiobiotin-fluorophosphonate for labeling of serine hydrolases prior to separation by SDS-PAGE and subsequent Western blot analysis to visualize resultant biotinylated proteins (C). A protein band was identified in which the intensity of labeling by desthiobiotin-fluorophosphonate correlated well with the observed PLA2 catalytic activity in each fraction (fractions 25–27 in the red dotted box in B). Hsp70, 70-kDa heat shock protein; MCAD, medium-chain acyl-CoA dehydrogenase; SCAD, short-chain acyl-CoA dehydrogenase.