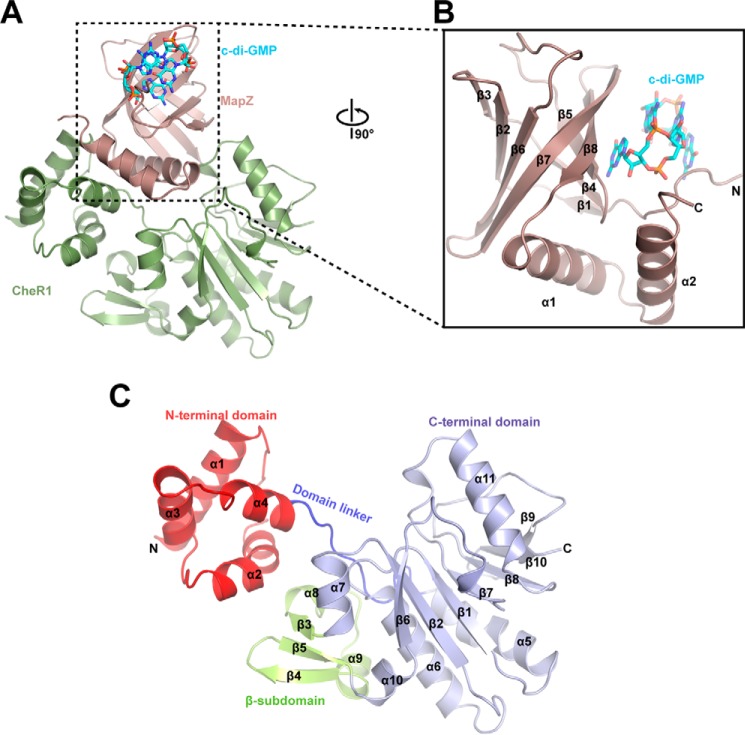
Figure 1.
Crystal structure of MapZ–c-di-GMP–CheR1 ternary complex. A, overall view of the ternary complex. MapZ and CheR1 are shown in salmon and green, respectively. The intercalated c-di-GMP dimer is bound by MapZ and shown as sticks with carbon, oxygen, and nitrogen atoms colored cyan, red, and blue, respectively. B, an enlarged view of c-di-GMP–bound MapZ. The view is rotated by 90° along the vertical axis in A. C, schematic diagram of CheR1. The N-terminal domain, domain linker, C-terminal domain, and β-subdomain are colored red, blue, light blue, and yellow, respectively. The protein secondary structures are numbered in sequential order from the N to C terminus.