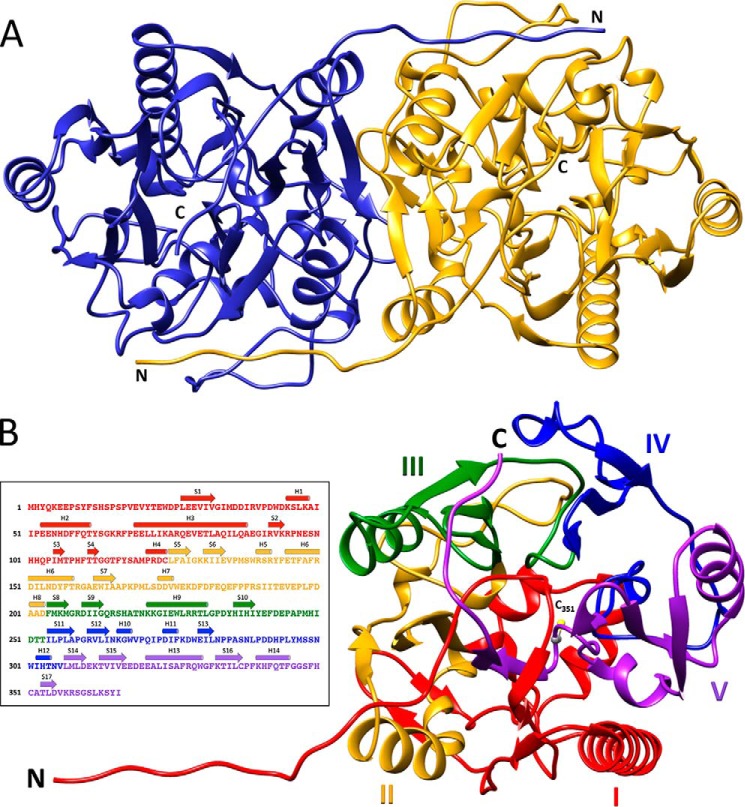
Figure 3.
Overall architecture of HsvA. A, ribbon diagram of the HsvA dimer. The monomers are related by a 2-fold symmetry axis at the dimer interface. The locations of the amino (N) and carboxyl (C) termini are indicated. B, domain organization of an HsvA monomer. The tertiary structure consists of five ββαβ modules arranged around a pseudo-5-fold axis, forming an α/β propeller. The modules are numbered I–V and are colored as follows: red, module I (residues 3–124); yellow, module II (residues 125–203); green, module III (residues 204–253); blue, module IV (residues 254–306); purple, module V (residues 307–366). The amino (N) and carboxyl (C) termini are identified, along with the position of the conserved nucleophilic cysteine in the active site (C351). The inset illustrates the position of secondary structural elements within the amino acid sequence, colored in the same manner as in the ribbon diagram. Helices (shown as cylinders) are numbered H1–H14, and β-strands (shown as arrows) are numbered S1–S17.