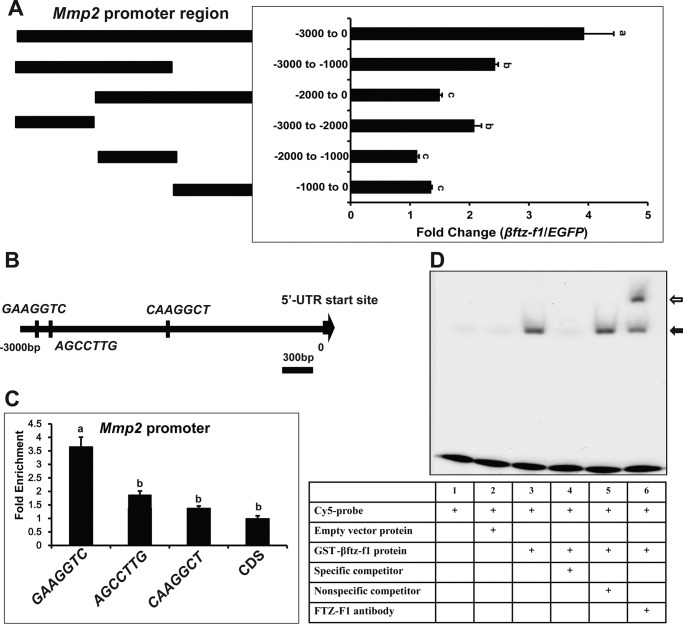
Figure 5.
Identification of a FBS in Mmp2 promoter. A, dual luciferase assay. Kc cells were co-transfected with a βftz-F1 expression construct (EGFP was used as a control), along with pGL3-basic plasmids containing Mmp2 promoter regions of different lengths. After 48 h of transfection, dual luciferase assays were performed. The luciferase activity fold change is defined as the relative luciferase activity induced by βftz-F1 overexpression compared with EGFP overexpression. B, locations of three βftz-F1 putative binding sites within the Mmp2 3-kb promoter. C, ChIP-qPCR. At 48 h after transfection, FLAG-βftz-F1-overexpressing Kc cells were fixed and subjected to ChIP using a FLAG mouse monoclonal antibody. Mock immunoprecipitations with preimmune serum were performed as negative controls. The precipitated DNA (different fragments in the 3-kb promoter region of Mmp2) and input were analyzed by qPCR to detect binding ability. D, EMSA. We used a three times repeated TGGGGGAAGGTCAAAT sequence (KBS), corresponding to a site located in the −2604 to −2595 region upstream of the Mmp2 transcription start site. A Cy5-labeled βftz-F1 binding site was added to a mixture with GST-βftz-F1 fusion proteins, in the presence (lane 4) or absence (lane 5) of an unmodified competitor. A supershift band was observed when a FTZ-F1 antibody was added into the reaction mixture.