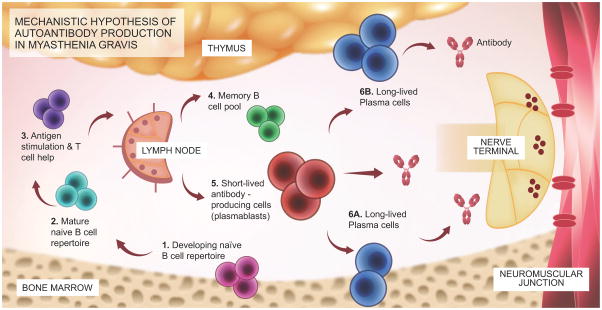
Figure 1.
Schematic diagram outlining the mechanistic hypothesis for the production of AChR or MuSK MG autoantibodies.
The proposed mechanistic path to autoantibody production in MG begins with naïve B cells (Steps 1 and 2), which likely encounter antigen(s) and receive T cell help in the lymph node (3). They then differentiate into memory B cells (4), antibody-secreting plasmablasts (5), and antibody-secreting long-lived plasma cells, which reside in the bone marrow (6A) and may also be present in the thymus (6B) of some patients with AChR MG. Plasmablasts and plasma cells may contribute to MG autoantibody production. B cell depletion therapy eliminates CD20+ memory and naïve B cells but does not directly eliminate plasmablasts or plasma cells, which are CD20-negative. After CD20-targeted depletion, MG serum autoantibody titers markedly diminish (especially in MuSK MG), suggesting that plasma cells are unlikely candidates for autoantibody production. Rather, short-lived plasmablasts are more viable candidates. As only a small fraction of these cells express CD20, the effectiveness of B cell depletion therapy may depend upon depletion of a pool of plasmablast-progenitor CD20+ memory B cells. Conversely, autoantibody titers that remain elevated following CD20-targeted depletion may be the product of long-lived plasma cells.