Abstract
BACKGROUND
Antibodies inhibiting the programmed death receptor 1 (PD-1) have shown significant activity in the treatment of advanced cutaneous melanoma. The efficacy and safety of PD-1 blockade in patients with uveal melanoma has not been well characterized.
METHODS
58 stage IV uveal melanoma patients received PD-1 or PD-L1 antibodies between 2009 and 2015 at nine academic centers. Patients who were evaluable for response were eligible for analysis. Imaging was performed every 12-weeks and at the investigators’ discretion. Safety and clinical efficacy outcomes including best overall response (BOR), progression-free survival (PFS), overall survival (OS) were determined retrospectively.
RESULTS
Of 56 eligible patients, 48 (86%) had received prior therapy and 35 (63%) had been treated with ipilimumab. Three patients had an objective response to ipilimumab and 8 patients had stable disease as the best response. 38 (68%) received pembrolizumab, 16 (29%) received nivolumab, and 2 (4%) received atezolizumab. Objective tumor responses were observed in two patients for an overall response rate of 3.6% (95% CI 1.8-22.5%). Stable disease (≥ 6 months) was observed in 5 (9%) patients. The median PFS was 2.6 months (95% CI 2.4-2.8 months) and the median OS was 7.6 months (95% CI 0.7-14.6 months). There was no association between prior treatment with ipilimumab or liver directed therapy and PFS or OS. Treatment was well tolerated and only 1 patient discontinued treatment due to toxicity.
CONCLUSIONS
PD-1 and PD-L1 antibodies rarely confer durable remissions in patients with metastatic uveal melanoma. Clinical trial enrollment should be prioritized in this population.
Keywords: uveal melanoma, immunotherapy, nivolumab, pembrolizumab, atezolizumab
INTRODUCTION
Uveal melanoma is the most common primary adult malignancy of the eye,1 with 2580 new cases and 270 deaths in the United States annually.2 Although uveal melanoma shares histologic features with cutaneous melanoma,3 it has distinct molecular and pathogenic features. While oncogenic BRAF and NRAS mutations are seen in 50% and 15% of cutaneous melanomas respectively,4 these mutations are rare in uveal melanoma tumors. Instead, 90% harbor mutations in either the GNAQ or GNA11 components of the guanine nucleotide binding protein subunit alpha.5,6 Uveal melanoma also has a distinct propensity to metastasize to the liver, with 80 to 90% of metastatic uveal melanoma patients ultimately exhibiting hepatic involvement.7,8 Although available clinical data are limited, metastatic uveal melanoma is refractory to many conventional therapies7,9 and the median overall survival (OS) for patients with this condition remains limited. The MEK inhibitor selumetinib induces objective tumor response in 14% of patients, but the median progression free survival (PFS) for uveal melanoma patients treated with this agent was only 4 months. This same phase II randomized trial failed to establish a survival benefit for selumetinib compared with chemotherapy.10,11 In this context, additional effective treatment options are urgently needed.
Immune checkpoint inhibitors induce antitumor immune responses by disinhibiting native immunity. To date, the Food and Drug Administration (FDA) has approved three immune checkpoint inhibitors for the treatment of unresectable or metastatic melanoma. Ipilimumab, an antibody against cytotoxic T-lymphocyte associated protein 4 (CTLA-4), blocks immune inhibitory interactions between CTLA-4 and B7. Nivolumab and pembrolizumab, antibodies targeting programmed cell death receptor 1 (PD-1), inhibit interactions between this receptor and its ligand (PD-L1). These agents have revolutionized the care of patients with advanced cutaneous melanoma, and also induce durable objective responses in a wide spectrum of malignances, including non-small cell lung cancer,12,13 transitional cell carcinoma of the bladder,14 renal cell carcinoma,15 squamous cell carcinoma of the head and neck,16 and Hodgkin’s lymphoma17 with objective response rates ranging from under 20% to over 60%. Objective responses to single-agent PD-1 antibody therapy appear to be most common in tumors with pre-treatment tumor infiltration with cytotoxic lymphocytes18 and intratumoral expression of markers of T cell exhaustion, including PD-L119.
Although these immune checkpoint inhibitors have shown substantial activity in advanced cutaneous melanoma, their role in uveal melanoma has not yet been defined. In cutaneous melanoma, ipilimumab induces objective responses in 11-19% of patients, with a 2-year OS rate of 24%.19,20. Anti-PD-1 monotherapy with pembrolizumab or nivolumab confers objective responses and durable remissions in 30-40% of cutaneous melanoma patients, with a favorable toxicity profile.21,22,23 In contrast, none of 53 uveal melanoma patients treated with ipilimumab in the largest phase II trial to date had objective responses, and the 2-year OS rate was much lower at 7%.24 Also, only two objective responses to ipilimumab were noted in an earlier retrospective study of 39 metastatic uveal melanoma patients.25 Uveal melanoma patients were included in several of the initial studies of PD-1 antibodies, but data are limited because these patients were excluded from most subsequent clinical trials. A recent case series described two objective responses in metastatic uveal melanoma patients treated with pembrolizumab as part of an expanded access study.26 To investigate this further, we report clinical outcomes in a larger series of 56 patients treated at nine institutions with PD-1 (pembrolizumab or nivolumab) or PD-L1 (atezolizumab) antibodies.
METHODS
Study population
Clinical data were collected from 58 patients across nine institutions in the United States and Spain. Participating institutions included the University of California, San Francisco (n=13); Memorial Sloan-Kettering Cancer Center (n=9); the University of California, Los Angeles (n=6); the Multidisciplinary Melanoma Group of Spain (n=5); Dana Farber Cancer Center (n=2); Vanderbilt University (n=3); Columbia University (n=2); the Moffitt Cancer Center (n=8); and Massachusetts General Hospital (n=10). Eligible patients were defined as those with stage IV uveal melanoma receiving therapy with a PD-1 (pembrolizumab or nivolumab) or a PD-L1 antibody (atezolizumab, MPDL3280A) between October 2009 and May 2015 with baseline imaging and follow-up data. Complete follow-up data were defined as 12-week follow-up imaging available for review by the participating institution, or if no follow-up imaging was available, documentation of death within 16 weeks of the initiation of treatment. Demographics, treatment parameters, and clinical outcomes were reported by the investigators at participating institutions and the data were analyzed at the University of California, San Francisco. Participating investigators reported response data using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.127 and toxicities were reported using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.028.
Assessments
Clinical data retrieved from electronic medical records included: age, sex, sites of metastatic disease at treatment initiation, baseline Eastern Cooperative Oncology Group (ECOG) performance status, prior systemic regimens, and response to prior therapies (including ipilimumab). Participating investigators also reported the specific PD-1 or PD-L1 antibody used, the dose, the dosing schedule, and mutation analysis results (for GNAQ, GNA11, and/or BRAF) where available. Best overall response, PFS, and OS data were calculated using baseline and follow-up imaging assessments made by the participating investigators. Additional data collected included the causes of treatment discontinuation, treatment regimens post-progression, and clinically significant adverse events.
Statistical Analysis
Patient demographics, treatment, and treatment history data were tabulated and reported descriptively. Liver-directed therapies such as chemoembolization and radiofrequency ablation were tabulated and reported separately from systemic therapy data. The reported best overall response was also tabulated. “Stable disease” was defined as a progression free interval of 6 months or longer in the absence of an objective tumor response. PFS was calculated from the date of initiation of anti-PD-1 therapy to the date of radiographic progression, death or last follow up. OS was estimated from the date of initiation of anti-PD-1 therapy to the date of death or last follow up. PFS and OS were estimated using the Kaplan-Meier method and expressed as median values with 95% confidence intervals.29 Univariate analysis using the log-rank test was performed for factors influencing PFS and OS, with p-values < 0.05 considered significant. Adverse event reporting using the CTCAE was based on available data at participating institutions. All statistical analysis was done using SPSS version 23.
RESULTS
Baseline demographics and clinical characteristics
Two patients were not eligible due to the absence of appropriate follow-up imaging and were excluded from analysis. Demographic and clinical data from the remaining 56 eligible patients are summarized in Table 1. The median age of these patients at the time of diagnosis with primary uveal melanoma was 55.5 years (range 25.8 to 84.1 years) and the median age at the time of initiation of PD-1 or PD-L1 antibody therapy was 62.4 years (range 38.2 to 88.6 years). 32 of 56 patients (57.1%) were male, and 52 (92.9%) had an ECOG performance status of 0 or 1. Similar to previous reports, 89.3% of patients had liver metastases. Other common sites of metastases included the lung (41.1%), bone (25%), lymph nodes (19.6%), and the central nervous system (12.5%). Thirteen patients (23.2%) received prior liver directed therapy and 48 (85.7%) received prior systemic therapy in the metastatic setting. Of those who had received prior systemic therapy, 28 (50%) patients had received one prior regimen and 20 (35.7%) had received two or more prior regimens. Thirty-five (62.5%) patients had received ipilimumab prior to receiving a PD-1 or PD-L1 antibody. Of these, 3 patients had objective responses to ipilimumab, and 8 had stable disease as the best response.
Table 1.
Baseline demographics and clinical characteristics
| Characteristic | Uveal melanoma patients (N=56) |
|---|---|
| Median age at diagnosis, years (range) | 55.5 (25.8–84.1) |
| Median age at initiation of PD-1 Ab therapy, years (range) | 62.4 (38.2–88.6) |
| Sex – no. (%) | |
| Male | 32 (57.1) |
| Female | 24 (42.9) |
| LDH above institutional ULN (no. %) | 40 (71.4%)1 |
| Baseline tumor burden2 | |
| Mean baseline tumor burden (range) | 10.6 cm (0–37.6) |
| Mean baseline tumor burden, liver only (range) | 7.2 cm (0–27.9) |
| Metastatic sites – no. (%)3 | |
| Liver | 50 (89.3) |
| Lung | 23 (41.1) |
| Bone | 14 (25.0) |
| Lymph nodes | 11 (19.6) |
| CNS | 7 (12.5) |
| Other | 22 (39.3) |
| ECOG performance status – no. (%) | |
| 0 | 25 (44.6) |
| 0–1 | 5 (8.9) |
| 1 | 22 (39.3) |
| 2 | 4 (7.1) |
| Number of prior systemic regimens – no. (%) | |
| 0 | 8 (14.3) |
| 1 | 28 (50.0) |
| 2 | 17 (30.4) |
| 3 | 1 (1.8) |
| ≥4 | 2 (3.6) |
| Best response to prior ipilimumab – no. (%) | |
| CR | 1 (1.8) |
| PR | 2 (3.6) |
| SD | 8 (14.3) |
| PD | 22 (39.3) |
| No prior ipilimumab | 21 (37.5) |
| Prior liver-directed therapy – no. (%)4 | 13 (23.2) |
| Bland embolization | 3 (5.4) |
| Chemoembolization | 5 (8.9) |
| Immunoembolization | 1 (1.8) |
| Radioembolization | 4 (7.1) |
| External beam radiation | 2 (3.6) |
| Radio frequency ablation | 1 (1.8) |
Abbreviations: LDH, lactate dehydrogenase; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease, ULN, upper limit of normal.
Baseline LDH unavailable for 3 patients
Baseline tumor burden determined by sum of diameters of target lesions according to RECIST v1.1 guidelines.
Total will be >56 as many patients had more than one site of disease.
Three patients had 2 types of liver directed therapy
Treatment summary
The analysis included patients treated with the PD-1 antibodies pembrolizumab and nivolumab as well as the PD-L1 antibody atezolizumab. Many patients were treated on clinical trials at doses and on treatment schedules that differ from the FDA-approved doses of pembrolizumab (2 mg/kg every 3 weeks) and nivolumab (3 mg/kg every 2 weeks, Table 2). For example, while 27 (48.2%) patients were treated with pembrolizumab at 2 mg/kg every 3 weeks, 9 (16.1%) patients were treated with pembrolizumab at doses of 10 mg/kg every 2 or 3 weeks. Ten patients (17.9%) were treated with nivolumab at 3 mg/kg every 2 weeks, but 6 (10.7%) were treated at 1, 2, or 10 mg/kg. Two patients were treated at different doses of the PD-L1 antibody atezolizumab, which is not currently FDA-approved for the treatment of metastatic melanoma.
Table 2. Treatment regimens.
| Agent and Dose | Schedule | No. (%) (N=56) |
|---|---|---|
| Pembrolizumab | ||
| 2 mg/kg | Q3W | 27 (48.2) |
| 10 mg/kg | Q2W | 3 (5.4) |
| 10 mg/kg | Q3W | 6 (10.7) |
| Unknown | Q3W | 2 (3.6) |
| Nivolumab | ||
| 1 mg/kg | Q2W | 4 (7.1) |
| 2 mg/kg | Q2W | 1 (1.8) |
| 3 mg/kg | Q2W | 10 (17.9) |
| 10 mg/kg | Q2W | 1 (1.8) |
| Atezolizumab (MPDL3280A) | ||
| 10 mg/kg | Q2W | 1 (1.8) |
| 15 mg/kg | Q2W | 1 (1.8) |
Abbreviations: Q2W, every 2 weeks; Q3W, every 3 weeks
Efficacy analyses
Objective responses
Treatment outcomes are summarized in Table 3. In this cohort, 2 patients had PRs and there were no patients with CRs, for an ORR of 3.6% (95% CI 1.8 to 22.5%). Five (8.9%) patients had stable disease for at least six months as the best response, and 48 (86.7%) patients had PD including 8 patients who died within 16 weeks with no available follow-up imaging. One patient had SD 5 months after starting treatment and was subsequently lost to follow-up. Figure 1 illustrates the percent change in disease burden from baseline in the 48 patients with available follow-up imaging.
Table 3. Response to treatment with PD-1 and PD-L1 antibodies.
| Best overall response1, 2 | No. (%) (N=56) |
|---|---|
| Complete response | 0 |
| Partial response | 2 (3.6) |
| Stable disease ≥ 6 months | 5 (8.9) |
| Progressive disease | 48 (85.7) |
The best overall response was assessed by the investigator with the use of the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
Response not included for a patient who was lost to follow-up with stable disease at 5 months.
Figure 1. Best overall responses.
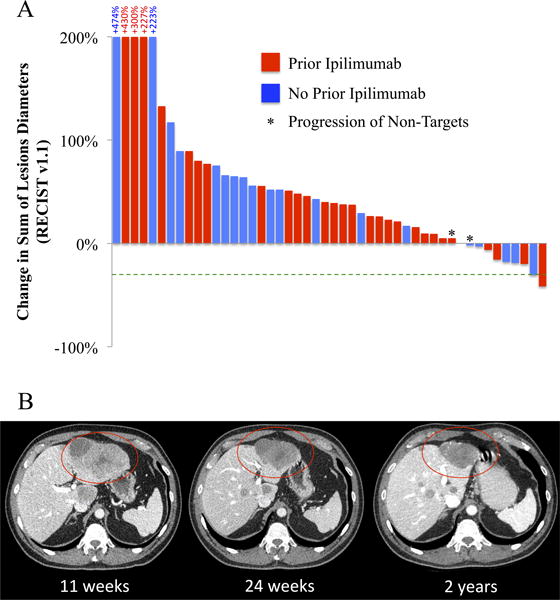
Best overall response data assessed as the greatest percent change in tumor diameters available for 48 patients. 8 patients who died within 16 weeks without available follow-up images are not included. 39 patients had a net increase in the sum of tumor diameters or progression of non-target lesions as the best response (Panel A). Serial imaging of a liver mass in a 61 year-old man with metastatic uveal melanoma responding to pembrolizumab (Panel B).
Two patients had objective partial responses to treatment. A 66-year-old woman with metastatic uveal melanoma involving the liver, bone, perineum, and subcutaneous tissues had been previously treated with the protein kinase C inhibitor AEB071 with disease progression as the best response and liver-directed bland embolization. At baseline, her lactate dehydrogenase (LDH) was more than two times the institutional upper limit of normal, and her overall sum of target lesion diameters was 13.9 cm (mean 10.6 cm for this cohort) and 2.5 cm in the liver (mean 7.2). She had a 31% reduction in the sum of tumor diameters 16 months after initiation of treatment with nivolumab at a dose of 3 mg/kg every 2 weeks. She remains on treatment and in response after 23 months and 46 cycles of therapy. A 61-year-old man with metastatic uveal melanoma isolated to the liver had stable disease with two prior regimens: carboplatin/paclitaxel/axitinib and ipilimumab. He had not received any prior liver-directed therapies. His baseline LDH was below the institutional upper limit of normal, and his baseline tumor burden in the liver was 9.1 cm overall, with no extra-hepatic lesions noted. Eleven weeks after the initiation of pembrolizumab, he had significant disease progression but therapy was continued. After 24 weeks of therapy, his tumors had decreased substantially in size and he achieved an objective response within 8 months. He currently remains on treatment with a 41.7% reduction in the sum of tumor diameters after 39 cycles and 27.4 months of pembrolizumab (Figure 2B).
Figure 2. PFS and OS estimates.
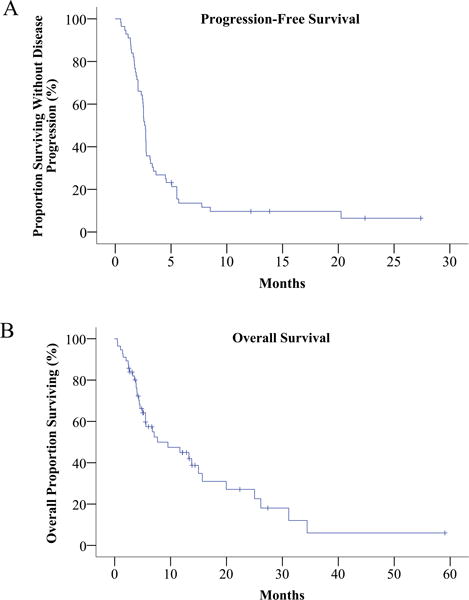
Kaplan-Meier curves showing progression free survival (Panel A) and overall survival (Panel B) in all 56 patients.
Five (8.9%) patients achieved stabilization of disease lasting for at least 6 months (range 7.5 to 20.3 months) from the initiation of therapy. These patients included a 75-year-old woman previously with stable disease on paclitaxel and disease progression on both bevacizumab and sunitinib who ultimately progressed after 7.8 months on nivolumab; a 38-year-old man with metastatic disease in the lungs who previously progressed on high dose IL-2 but remained progression-free for over 20 months on pembrolizumab; a 39-year-old man with liver metastases and prior disease progression on ipilimumab who remained progression-free for 8.5 months on pembrolizumab; a 56-year-old woman with liver, lung, and orbital involvement and prior stable disease progression on ipilimumab who remains progression-free after 12 months on pembrolizumab; and a 45-year-old man with lung, peritoneal, and brain metastases who previously progressed on ipilimumab but who currently remains progression-free after 13.8 months on pembrolizumab.
Clinical Benefit and Prior Immune Therapy
Prior immune therapy in patients benefiting from PD-1 antibody therapy
One of the patients achieving a partial response to pembrolizumab had previously had disease stabilization when treated with ipilimumab. However, two patients with stable disease lasting for over 6 months on PD-1 antibodies had not had clinical benefit from ipilimumab. Another patient with stable disease for over 20 months on pembrolizumab previously progressed on high dose interleukin-2.
PD-1 antibody therapy outcomes in patients with prior response to ipilimumab
Two patients who previously had responses to ipilimumab progressed on pembrolizumab. A third patient with a reported complete response to ipilimumab followed by disease progression remains on pembrolizumab without disease progression or objective response 5 months after starting treatment.
Progression Free Survival
The median PFS across the entire cohort was 2.6 months (95% CI 2.4-2.8 months). Five patients remain progression-free with a median follow up of 13.8 months. There was no significant difference in PFS associated with age (over or under 60 years), or the presence or absence of liver or lung metastases. There was no association between PFS and prior response or exposure to ipilimumab, or between PFS and prior exposure to liver-directed therapy. PFS was significantly longer in women compared with men (median 3.2 vs 2.4 months, mean 6.2 vs 3.8 months, p < 0.05) and in patients with a normal baseline LDH compared with patients with elevated baseline LDH (median 2.8 vs 2.5 months, mean 10.5 vs 3.0 months, p < 0.05). Baseline LDH data were not available for 3 patients.
Overall Survival
The median OS across the entire cohort was 7.7 months (95% CI 0.7-14.6 months) and 20 patients were alive at the time of data analysis. On univariate analysis, there was no significant association between improved OS and age, or the presence or absence of liver or lung metastases. There was no association between OS and prior response or exposure to ipilimumab or between OS and prior exposure to liver directed therapy. OS was significantly longer in women compared with men (median 13.3 vs 5.0 months, mean 21.7 vs 11.0 months, p < 0.05) and in patients with a normal baseline LDH compared with patients with elevated baseline LDH (median 25.0 vs 5.2 months, mean 24.9 vs 11.9 months, p < 0.05). Baseline LDH data were not available for 3 patients.
Treatment Post-Progression
Of 51 patients who progressed on PD-1 or PD-L1 antibodies, 22 were able to pursue additional therapy after these progression events (Table 4). Twelve (21.4%) patients received molecularly targeted therapy and/or cytotoxic chemotherapy. Ten (17.9%) patients received ipilimumab monotherapy. One patient received nivolumab with ipilimumab and another patient received a second PD-1 antibody. Response data for these patients were not available at the time of data analysis.
Table 4. Discontinuation and post –PD-1 course.
Reasons given for PD-1 antibody or PD-L1 antibody discontinuation (Panel A). Treatment regimens administered after PD-1 or PD-L1 antibody discontinuation (Panel B).
| Subsequent therapy1 | No. (%) (N=56) |
|---|---|
| Alternative PD-1 antibody | 1 (1.8) |
| Ipilimumab monotherapy | 10 (17.9) |
| Nivolumab + ipilimumab | 1 (1.8) |
| Molecularly targeted therapy and/or chemotherapy | 12 (21.4) |
| Liver-directed therapy (embolization, radiation) | 4 (7.1) |
| Received no subsequent therapy or continuing on PD1 or PDL1 Ab | 34 (60.7) |
Total will be >56 as some patients received more than one type of subsequent therapy.
Safety
PD-1 or PD-L1 antibody therapy was well tolerated. No adverse events (AEs) were reported for 22 patients (37.9%). Grade 3 AEs were observed in 7 (12.5%) patients and included nausea, vomiting, hyperbilirubinemia, fatigue, colitis, arthralgia and lymphopenia (Table 5). No grade 4 or 5 AEs were reported. Only one of the 58 patients discontinued treatment due to toxicity (grade 3 arthralgia; Table 3A). The most common AEs of any grade were fatigue (19.6%), pruritus (12.5%), rash (10.7%), nausea (10.7%), hypothyroidism (7.1%), and diarrhea (8.9%).
Table 5. Investigator-reported adverse events.
| Adverse event | Grade 1 or 2 N (%) |
Grade 31 N (%) |
|---|---|---|
| Any adverse event | 25 (45.6) | 7 (12.5) |
| Constitutional | ||
| Fatigue | 10 (17.8) | 1 (1.8) |
| Anorexia | 4 (7.1) | |
| Fever | 3 (5.4) | |
| Infusion reaction | 2 (3.6) | |
| Night sweats | 2 (3.6) | |
| GI | ||
| Nausea | 5 (8.9) | 1 (1.8) |
| Diarrhea | 5 (8.9) | |
| Dry mouth | 3 (5.4) | |
| Colitis | 0 | 2 (3.6) |
| Vomiting | 1 (1.8) | 1 (1.8) |
| Pancreatitis | 1 (1.8) | |
| Skin | ||
| Pruritus | 7 (12.5) | |
| Rash | 6 (10.7) | |
| Hyperhidrosis | 1 (1.8) | |
| Vitiligo | 1 (1.8) | |
| Endocrine | ||
| Hypothyroidism | 4 (7.1) | |
| Thyroiditis | 1 (1.8) | |
| Hypophysitis | 1 (1.8) | |
| Pain | ||
| Back pain | 2 (3.6) | |
| Abdominal pain | 2 (3.6) | |
| Extremity pain | 1 (1.8) | |
| Bone pain | 1 (1.8) | |
| Laboratory | ||
| Hyperbilirubinemia | 0 | 1 (1.8) |
| Elevated ALP | 1 (1.8) | |
| Elevated ALT | 1 (1.8) | |
| Anemia | 1 (1.8) | |
| Lymphopenia | 1 (1.8) | |
| Respiratory | ||
| Cough | 2 (3.6) | |
| Dyspnea | 1 (1.8) | |
| Musculoskeletal | ||
| Arthralgia | 1 (1.8) | 1 (1.8) |
| Ocular | ||
| Eye irritation | 1 (1.8) | |
| Eye disorder NOS | 1 (1.8) | |
| No adverse events reported | 22 (39.3) |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; NOS, not otherwise specified
No grade 4 or 5 adverse events were reported.
Molecular analysis
GNAQ and GNA11 mutation analysis was available for 15 patients. Eight of these patients had tumor harboring a GNAQ mutation, and 6 patients had GNA11 positive tumors. One patient had no detectable exon 5 GNAQ or GNA11 mutations, and an additional patient was found to be negative for GNAQ mutations with no record of GNA11 mutation analysis. None of the patients with PD or SD after treatment with PD-1 or PD-L1 antibodies were tested for GNAQ or GNA11 mutations. None of seven patients tested for BRAF exon 15 mutations harbored such mutations.
DISCUSSION
In the largest case series of uveal melanoma patients treated with PD-1 or PD-L1 antibodies to date, the objective response rate, median PFS, and median OS were substantially lower than seen in cutaneous melanoma.21–23 Objective tumor responses in uveal melanoma patients treated with PD-1 antibodies have been reported previously in a small case series of 7 patients.30 The data reported here confirm that PD-1 and PD-L1 antibodies can confer clinical benefits in uveal melanoma patients (CBR in 7 of 56, or 12.5% of patients), but these responses are rare, with durable partial responses observed in 3.6% of patients in our experience. Furthermore, the median PFS (2.6 months) and OS (7.7 months) were also modest, demonstrating that major therapeutic benefits of PD-1/PD-L1 antibody therapy were limited to a small subset of patients. As in patients with other advanced solid tumors, PD-1 and PDL-1 antibodies were well tolerated; only one of the 56 patients in this series discontinued treatment due to toxicity. These data suggest that the preferred front-line therapy for advanced uveal melanoma should remain clinical trial participation. For patients who are cannot or who choose not to participate in clinical trials, anti-PD-1 therapy may be beneficial in a subset of patients.
As described in cutaneous melanoma,31 there did not seem to be a clear association between clinical benefit from PD-1 or PD-L1 antibodies and benefit from prior treatment with other immune therapies, including ipilimumab. These data are limited due to the few patients responding to any given immune therapy, but overall, the data presented here suggest that uveal melanoma can respond to immune checkpoint inhibitors. Although not all patients received ipilimumab prior to PD-1 or PD-L1 antibody therapy, five patients responded to either ipilimumab or PD-1 antibody therapy. Furthermore, durable responses in uveal melanoma to both PD-1 and CTLA-4 antibodies have now been described in multiple prospective and retrospective studies.24–26 These data as well as the absence of other highly effective treatment options suggest that further study of immune checkpoint inhibitor therapy in uveal melanoma is warranted.
Although this clinical series is the largest thus far investigating outcomes of uveal melanoma patients treated with PD-1 and PD-L1 antibodies, the retrospective nature of this analysis limits the scope and generalizability of the results. For example, there were significant variations in the agents, dosing and treatment schedules used. It is unlikely, however, that these variations negatively influenced clinical outcomes. Although individual patients were treated with different agents according to different treatment schedules, at least 36 of 38 patients treated with pembrolizumab and 11 of 16 patients treated with nivolumab received drug exposures that were equal to or greater than the approved regimens for these agents. Furthermore, available data suggest that overall response rates to PD-1 antibodies are similar across doses levels and administration schedules32,33. The limited activity of PD-1 and PD-L1 antibodies in our series may have been influenced by the relatively advanced stage of disease in the current, unselected uveal melanoma patient population. Over 70% of the patients of this population had an elevated LDH at baseline and this was a predictor of worse progression-free and overall survival. In addition, eight patients died within 16 weeks of the initiation of treatment, suggesting that they may have had aggressive, treatment-refractory disease at baseline. Controlled prospective testing of pembrolizumab is currently ongoing in a phase 2 clinical trial (NCT0235851).34
Given the low clinical benefit rate in uveal melanoma patients treated with PD-1 and PD-L1 antibodies, patient selection based on predictive biomarkers could be extremely useful clinically. Descriptive and predictive biomarker data were limited in the current retrospective analysis. Due to limitations in available assays, baseline PD-L1 immunohistochemistry data were not available, and limited tumor genotyping data were obtained. In patients with metastatic cutaneous melanoma and other advanced malignancies, tumors expressing high levels of PD-L1 (i.e., “PD-L1 positive”) are more likely to respond to anti-PD-1 monotherapy than those with low expression (i.e., “PD-L1 negative”).35 A PD-L1 expression assay has recently become commercially available36 and more comprehensive assays investigating the tumor microenvironment (i.e., understanding the abundance and relative locations of effector and regulatory T cells and the clonality of the T-cell repertoire18) may have improved positive and negative predictive value for determining response to PD-1 blockade. These assays could also determine which uveal melanoma patients could benefit from combination immune therapy. Although there are no published reports regarding the efficacy of ipilimumab administered concurrently with nivolumab in uveal melanoma patients, combined CTLA-4 and PD-1 immune checkpoint inhibition appears to be particularly beneficial in patients with PD-L1 negative tumors with low numbers of tumor infiltrating lymphocytes.18,20 Thus, assessment of the immune milieu in uveal melanoma may help to determine whether combination immune therapy would be worth evaluating prospectively in this population. PD-L1 and other biomarker analysis is an integral part of the ongoing prospective trial of pembrolizumab in uveal melanoma (NCT0235851).34
Future therapeutic advances may stem from a better understanding of immune evasion in uveal melanoma. Uveal melanoma harbors an extremely low somatic mutation burden37 and it possible that it is less responsive to immune checkpoint inhibition because it is inherently less antigenic. Although the overall tumor mutation burden has been associated with response to ipilimumab, it has also been suggested that the presence of immunogenic neoantigens may contribute to predicting benefit to this agent.38 However, more recent studies failed to confirm the role of specific neoantigens but did reconfirm the critical role of over tumor burden based on whole exome sequencing39,40. An immune-suppressive tumor microenvironment may also limit the activity of PD-1 and PD-L1 antibodies in uveal melanoma. Due to the role of the liver in filtering microbial products arriving from the gut, it has been suggested that the liver has enhanced tolerance to tumor antigens compared with other organs.41 Recent data suggest that the presence of liver metastases is associated with a lower response rate in melanoma patients treated with pembrolizumab,42 and, as in previously published reports, 80 to 90% of patients in this series had liver involvement7,8.
CONCLUSIONS
In the largest retrospective series thus far of clinical outcomes in metastatic uveal melanoma patients treated with PD-1 and PD-L1 antibodies, durable objective tumor responses and sustained disease control occurred but were rare. These results suggest that agents targeting the PD-1 axis are beneficial in a small subset of uveal melanoma patients, but further prospective studies are needed to confirm the low response rate, validate predictive biomarkers, and to identify the mechanisms of tumor response and progression in this population. Once available, these data could help to identify alternative treatment approaches or combinations that would yield durable remissions in a greater proportion of uveal melanoma patients. Lastly, uveal melanoma may simply be a cancer which will not easily be targeted by immune therapy and which will require alternate approaches based on our evolving understanding of its biology.
Acknowledgments
Grant : P30 CA008748.
Research support: Bristol-Myers Squibb, Merck, and Genentech provided financial support for the conduct of the trials retrospectively analyzed in this manuscript.
Footnotes
Conflict of Interest: Alain Algazi serves as Principal Investigator at UCSF for studies supported by Merck and Bristol-Myers-Squibb. Katy Tsai reports no relevant disclosures. Douglas Johnson serves on advisory boards for Bristol-Myers-Squibb and Genoptix. Alexander Shoushtari serves as PI at MSKCC for studies supported by BMS and has served on the scientific advisory board for Vaccinex. Richard Carvajal serves on advisory boards for Merck and Genentech.
Author Contributions:
Study concept and design: Alain Algazi and Katy Tsai.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Alain Algazi and Katy Tsai.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Alain Algazi, Katy Tsai and Jimmy Hwang.
Obtained funding: Not applicable.
Administrative, technical, or material support: Not applicable.
Additional Contributions: Michelle Walsh, RN, assisted in the collection of data for patients treated at Columbia University Medical Center. Megan Othus, PhD provided advice regarding statistical analysis.
References
- 1.Marshall EC. Epidemiology of tumors affecting the visual system. Optom Clin Off Publ Prentice Soc. 1993;3(3):1–16. [PubMed] [Google Scholar]
- 2.Eye Cancer: Statistics. Cancer.Net. http://www.cancer.net/cancer-types/eye-cancer/statistics. Accessed October 20, 2015.
- 3.Weis E, Shah CP, Lajous M, Shields JA, Shields CL. The association between host susceptibility factors and uveal melanoma: a meta-analysis. Arch Ophthalmol Chic Ill 1960. 2006;124(1):54–60. doi: 10.1001/archopht.124.1.54. [DOI] [PubMed] [Google Scholar]
- 4.Colombino M, Capone M, Lissia A, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(20):2522–2529. doi: 10.1200/JCO.2011.41.2452. [DOI] [PubMed] [Google Scholar]
- 5.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363(23):2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(31):8076–8080. doi: 10.1200/JCO.2005.02.6534. [DOI] [PubMed] [Google Scholar]
- 8.Kath R, Hayungs J, Bornfeld N, Sauerwein W, Höffken K, Seeber S. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993;72(7):2219–2223. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Augsburger JJ, Corrêa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148(1):119–127. doi: 10.1016/j.ajo.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311(23):2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AstraZeneca provides update on selumetinib in uveal melanoma - AstraZeneca. https://www.astrazeneca.com/our-company/media-centre/press-releases/2015/astrazeneca-selumetinib-uveal-melanoma-oncology-22072015.html. Accessed November 11, 2015.
- 12.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 13.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 15.Plimack ER, Hammers HJ, Rini BI, et al. Updated survival results from a randomized, dose-ranging phase II study of nivolumab (NIVO) in metastatic renal cell carcinoma (mRCC) ASCO Meet Abstr. 2015;33(15_suppl):4553. [Google Scholar]
- 16.Seiwert TY, Burtness B, Weiss J, et al. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV–associated head and neck (H/N) cancer. J Clin Oncol. 2014;32:5s. (suppl; abstr 6011). http://meetinglibrary.asco.org/content/132361-144. Accessed June 29, 2014. [Google Scholar]
- 17.Moskowitz CH, Ribrag V, Michot J-M, et al. PD-1 Blockade with the Monoclonal Antibody Pembrolizumab (MK-3475) in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Preliminary Results from a Phase 1b Study (KEYNOTE-013) Blood. 2014;124(21):290–290. [Google Scholar]
- 18.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 22.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 23.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 24.Zimmer L, Vaubel J, Mohr P, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naïve patients with metastatic uveal melanoma. PloS One. 2015;10(3):e0118564. doi: 10.1371/journal.pone.0118564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119(20):3687–3695. doi: 10.1002/cncr.28282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kottschade LA, McWilliams RR, Markovic S, et al. The use of pembrolizumab for the treatment of metastatic uveal melanoma. ASCO Meet Abstr. 2015;33(15_suppl):9010. doi: 10.1097/CMR.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer Oxf Engl 1990. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2010 Jun; [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 30.The use of pembrolizumab for the treatment of metastatic uveal melanoma. J Clin Oncol. http://meetinglibrary.asco.org/content/148865-156. Accessed October 19, 2015.
- 31.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pembrolizumab in Treating Patients With Advanced Uveal Melanoma - Full Text View. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02359851?term=pembrolizumab+AND+uveal&rank=1. Accessed April 6, 2016.
- 35.Carbognin L, Pilotto S, Milella M, et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PloS One. 2015;10(6):e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quest Diagnostics Introduces Dako’s PD-L1 Companion Diagnostic for KEYTRUDA®, Merck’s Anti-PD-1 Therapy for Metastatic Non-Small Cell Lung Cancer Whose Tumors Express PD-L1 with Disease Progression On or After Platinum-Containing Chemotherapy. http://www.prnewswire.com/news-releases/quest-diagnostics-introduces-dakos-pd-l1-companion-diagnostic-for-keytruda-mercks-anti-pd-1-therapy-for-metastatic-non-small-cell-lung-cancer-whose-tumors-express-pd-l1-with-disease-progression-on-or-after-platinum-containing–300153548.html. Accessed October 26, 2015.
- 37.Furney SJ, Pedersen M, Gentien D, et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013;3(10):1122–1129. doi: 10.1158/2159-8290.CD-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doherty DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2015 Sep; doi: 10.1016/j.jaut.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Tsai KK, Loo K, Khurana N, et al. Clinical characteristics predictive of response to pembrolizumab in advanced melanoma. ASCO Meet Abstr. 2015;33(15_suppl):9031. [Google Scholar]


