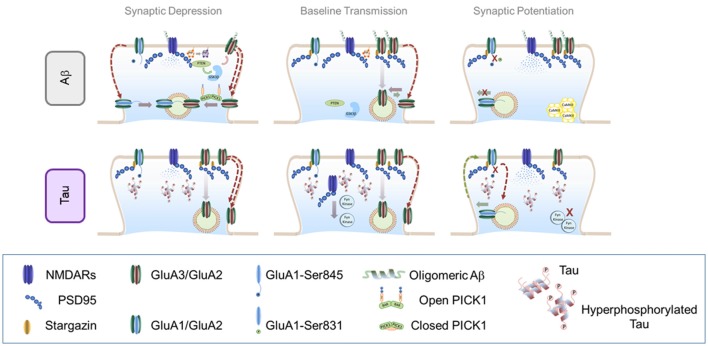
Figure 2.
Effect of soluble amyloid-β (Aβ) oligomers and hyperphosphorylated tau in AMPAR trafficking. Top panel: effect of soluble Aβ oligomers in AMPAR trafficking: Central panel: AMPAR trafficking during basal transmission: soluble Aβ oligomers may bind to NMDARs and AMPARs (preferentially to GluN2B and GluA2/GluA3 heteromers). This interaction enhances AMPAR endocytosis which decreases synaptic strength. Left Panel: AMPAR internalization during LTD: in the presence of Aβ, NMDAR-dependent LTD engages similar signaling pathways than regular LTD. However, endocytosis exacerbation may contribute to increase synaptic depression through a PICK1-dependent mechanism. Right panel: AMPAR insertion during LTP: accumulation of Aβ oligomers prevents CaMKII from reaching synaptic localizations and blocking AMPAR phosphorylation important for LTP expression. Bottom panel: effect of hyperphosphorylated tau in AMPAR trafficking. Central panel: AMPAR trafficking during basal transmission: hyperphosphorylated tau is missorted to dendritic spines where dysregulates key components of the PSD such as PSD95 and NMDARs. Hyperphosphorylated tau prevents Fyn kinase from reaching synaptic localizations which may alter basaline phosphorylation levels of both AMPARs and NMDARs. Left Panel: AMPAR internalization during LTD: as a consequence of reduced PSD stability, LTD may appear enhanced in the presence of hyperphosphorylated tau at synapses. Right Panel: AMPAR insertion during LTP: insertion of AMPARs may not be affected by hyperphophorylated tau, however subsequent synaptic retention may be impaired due to a less stable PSD. Additionally, the inability of critical protein kinases such as the Fyn kinase to reach their synaptic target may contribute to a loss of newly-inserted receptors.