Figure 1.
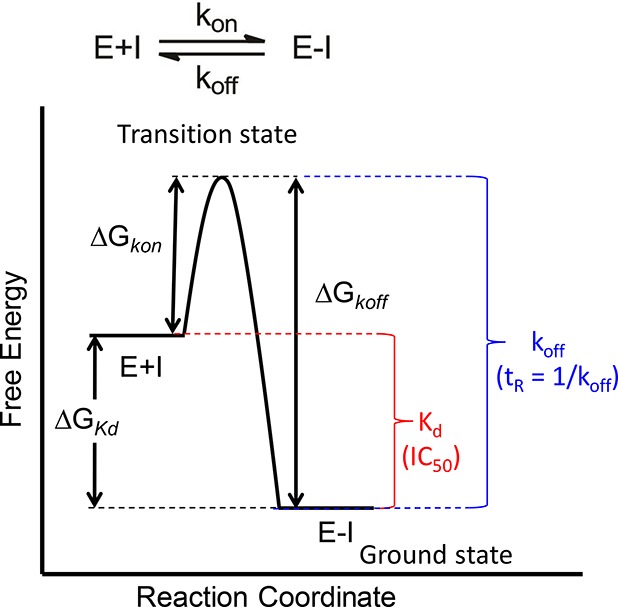
Reaction coordinate for a one-step binding event. Target (E) and drug (I) binding leads to the drug–target complex (E-I). The driving force for binding is given by the difference in free energy between E+I and E-I (ΔGKd). Experimental measurements of the equilibrium dissociation constant Kd, or parameters such as IC50 values, provide a quantitative estimate of the thermodynamics for binding. The rate at which the drug-target complex forms (kon) and dissociates (koff) is given by the difference in free energy between the respective ground states (E+I or E-I) and the rate-limiting transition state (ΔGkon and ΔGkoff). ΔGKd is related to Kd by the relationship ΔG° = −RT ln K. Assuming that parameters such as the transmission coefficient are the same for two drug molecules, then the difference in free energy for the rate of complex dissociation of the two molecules can be given by ΔΔGkoff = −RT ln(koff1/koff2). The lifetime of the drug–target complex is often quantified by the residence time, tR, where tR = 1/koff.5 The figure shows a simple one-step mechanism, although in many cases slow-binding inhibitors operate through a two-step induced-fit mechanism.13,15,28−31
