Figure 1.
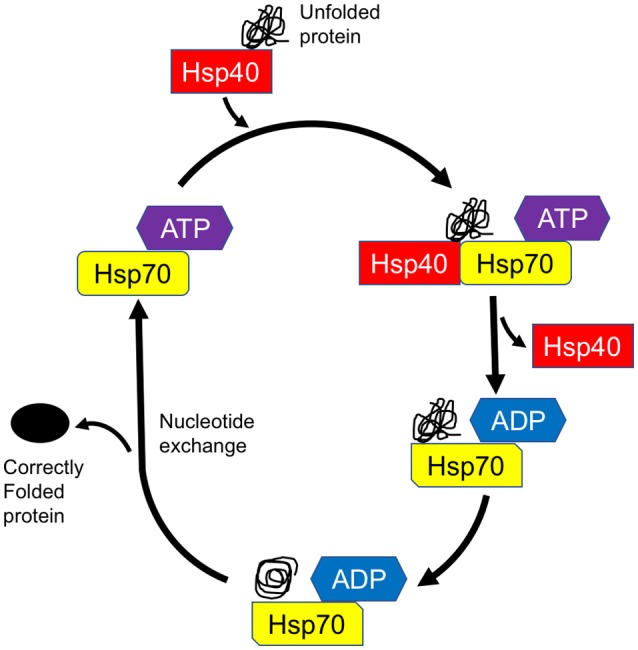
Model of chaperone-assisted protein folding by Hsp70-DnaJ/Hsp40 complex. DnaJ/Hsp40 transiently associates with unfolded protein (substrate) for delivery of the substrate to Hsp70. Through the intermediary of the J domain, Hsp40 binds to Hsp70 and promotes its ATPase activity, thus generating the ADP-bound Hsp70, which stably interacts with client protein. After nucleotide exchange, the substrate is released from Hsp70 and leaves the cycle as a correctly folded protein.
