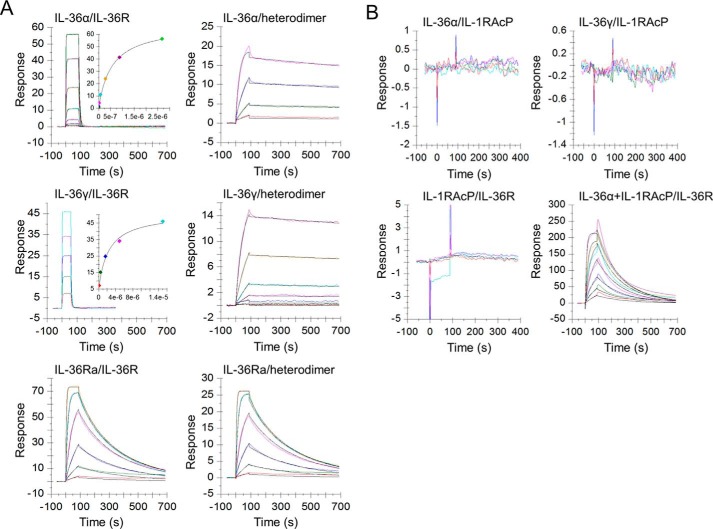
Figure 5.
IL-36 agonists and the antagonist bind to receptor IL-36R and heterodimer with distinct kinetics. A, left panels, agonists IL-36α/γ bind to IL-36R with fast-on and fast-off rates, whereas antagonist IL-36Ra dissociates from bound IL-36Ra·IL-36R complex at a slower off rate. Right panels, agonists IL-36α/γ bind to Fc-linked IL-36R·IL-1RAcP heterodimer and dissociate from it at a much slower rate than IL-36Ra. B, top panels, little or no IL-36α/γ binding to IL-1RAcP. Bottom panels, IL-1RAcP binds to IL-36R only when IL-36α is present. One representative sensorgram from at least two independent experiments is shown. Sensorgrams are colored lines, whereas fits are in black. Sensorgrams are globally fit using a 1:1 kinetics model with a mass transfer term included. Binding kinetics of IL-36γ·IL-36R is out of instrument limit. Affinity values for IL-36γ·IL-36R are obtained using steady-state fit.