Figure 7.
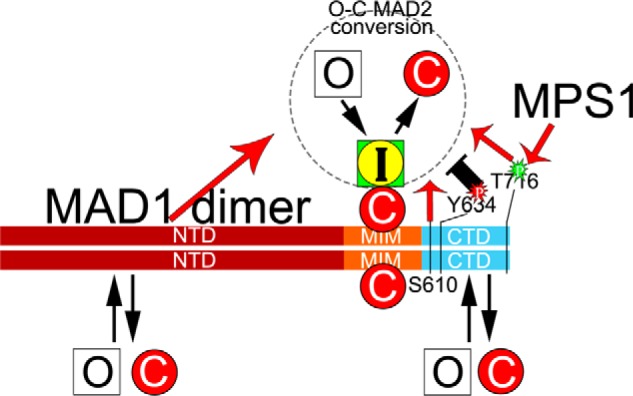
An updated model on the MAD1:C-MAD2 catalyst for MAD2 O–C conversion. In the classical model, a MAD1 dimer tightly binds two C-MAD2 molecules at its MIM region to become the catalyst for MAD2 O–C conversion. Our work has shown that MAD1NTD and MAD1CTD have additional, probably weaker, binding sites for both O-MAD2 and C-MAD2. MAD1NTD and MAD1CTD positively contribute to the MAD2 O–C conversion (red arrows), based on live cell imaging results. Several residues within the CTD domain may have important functional roles. Thr-716 phosphorylation by MPS1 and Ser-610 are required for full MAD1 activity. Tyr-634 might be a residue whose phosphorylation negatively impacts the O–C conversion. Similarly, the NTD:CTD interaction (not shown) may restrain the catalytic efficiency of MAD1, but the interaction can be disrupted by action of MPS1 kinase. It remains unclear whether the two C-MAD2 molecules bound to the MIM regions of the MAD1 dimer are equally engaged in MAD2 O–C conversion.
