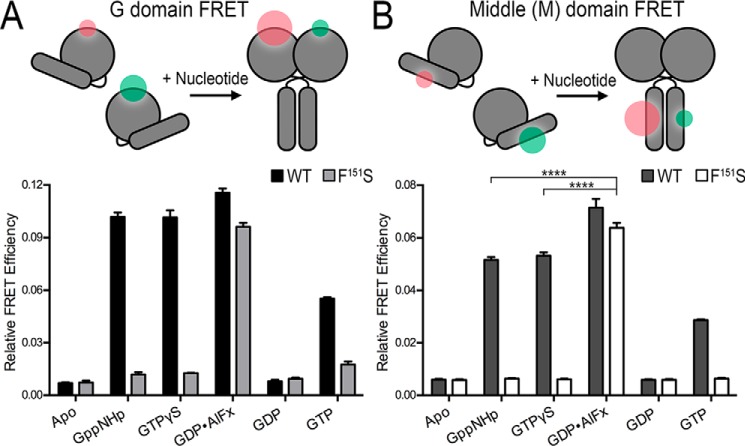
Figure 3.
ATL1-F151S utilizes both the G and middle domains in the transition-state dimer. A, equilibrium and steady-state G domain FRET dimerization. Both wild-type and ATL1-F151S proteins were labeled site-specifically (K295C) with donor (Alexa Fluor 488) and acceptor (Alexa Fluor 647) FRET fluorophores, and measurements including 1 μm protein and 500 μm nucleotide were taken once equilibrium (GTPγS, Gpp(NH)p, GDP, or GDP·AlFx) or steady-state (GTP) conditions were fulfilled. B, equilibrium and steady-state middle domain FRET dimerization. Analogous FRET measurements were conducted with wild-type and ATL1-F151S proteins labeled site-specifically at their middle domains (K400C). ATL-F151S has a statistically higher FRET efficiency than wild-type bound to non-hydrolyzable analogs (****, p ≤ 0.0001). Graphs showing means and S.D. (error bars) are plotted from two biological replicates with three technical repeats each. Sizes of the green and red halos in the schematic diagrams (top panels) illustrate the fluorophore intensities in FRET and non-FRET states.