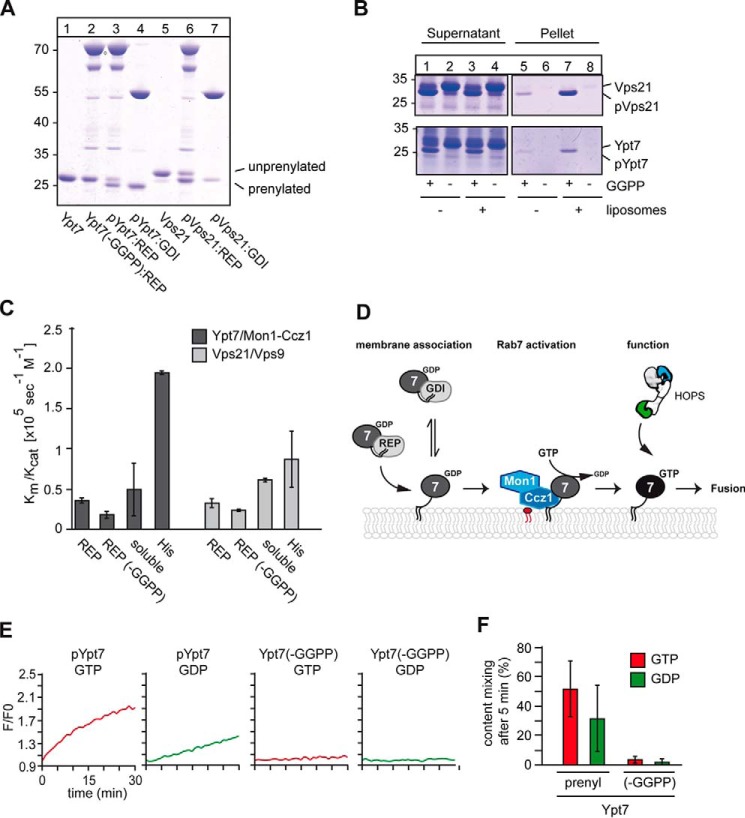
Figure 1.
Establishment of in vitro prenylation and functionality of Ypt7 and Vps21. A, prenylation of Ypt7 and Vps21 in vitro. 3 μm Ypt7 or Vps21, respectively, 3 μm REP (Mrs6), 1 μm geranylgeranyltransferase (GGTase, Bet2-Bet4) were incubated with or without geranylgeranyl pyrophosphate (GGPP) at 30 °C for 30 min. Rab–GDI complexes were obtained as described previously (10). Samples were analyzed by SDS-PAGE and Coomassie staining. B, membrane association of Rabs after prenylation. Vps21 (top) and Ypt7 (bottom) were incubated with the prenylation machinery in either the presence or absence of GGPP as described in A. Where indicated, liposomes were added. To allow for membrane insertion after prenylation, the samples were incubated for another 30 min at 30 °C and then centrifuged for 30 min at 20,000 × g. The supernatant and pellet were analyzed by SDS-PAGE and Coomassie staining. C, analysis of GEF activity. 50 pmol of MANT–GDP–loaded Rabs were incubated with the respective GEF as described under “Materials and methods.” Loss of fluorescence was monitored in a plate reader after addition of GTP to a final concentration of 0.1 mm for 30 min at 30 °C. GEF activity of the corresponding GEF toward prenylated (REP) and unprenylated (REP (−GGPP)) Rab–REP complexes was measured in the presence of liposomes. GEF assays with soluble Rabs were performed in the absence of liposomes using the corresponding GEF. C-terminally His-tagged Ypt7 and Vps21 (His) were incubated with their respective GEF in the presence of liposomes carrying DOGS-NTA. A summary of kcat/Km values was obtained from three different experiments. D, model of Mon1–Ccz1–dependent Rab recruitment to membranes. For details see text. E and F, analysis of RPL fusion. 160 nm prenylated or non-prenylated Ypt7–REP complex was added in the presence of 100 nm Mon1–Ccz1 and either GDP or GTP prior to starting fusion reactions to dye-loaded proteoliposomes. Proteoliposomes carried either the vacuolar SNAREs Vam3 and Vti1 or the R-SNARE Nyv1. The SNARE:lipid ratio was 1:10,000 (see “Materials and methods”). Fusion reactions were carried out in a plate reader for 30 min at 27 °C in the presence of a fusion mixture containing HOPS, Sec17, Sec18, Vam7, and ATP. After addition of the fusion mixture to each reaction, the FRET signal as a result of content mixing was recorded (n = 3).