Scheme 1. Schematic Diagram Showing the Electronic Configuration (α- and β-MOs) of a Neutral Parent Molecule and Its One-Electron Oxidized Radical.
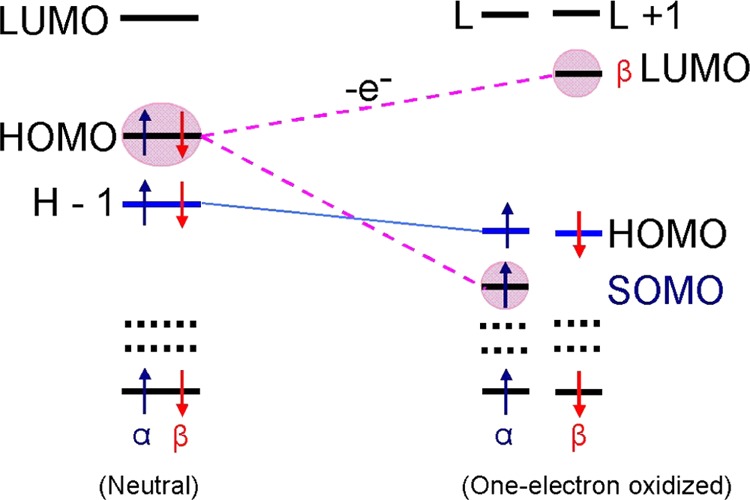
In the neutral molecule, each MO is doubly occupied; however, on one-electron oxidation (removal of an electron), α- and β-MOs rearranged independently. For all of the molecules described in this work, removal of an electron from HOMO of a neutral molecule splits HOMO of neutral molecule into β-LUMO and α-SOMO, with the SOMO buried in the filled orbitals by one to three levels. Note, as expected, the SOMO and the β-LUMO have near identical wave functions. HOMO = highest occupied molecular orbital; LUMO = lowest unoccupied molecular orbital; and SOMO = singly occupied molecular orbital. Blue and red arrows represent α and β spin of an electron, respectively.
