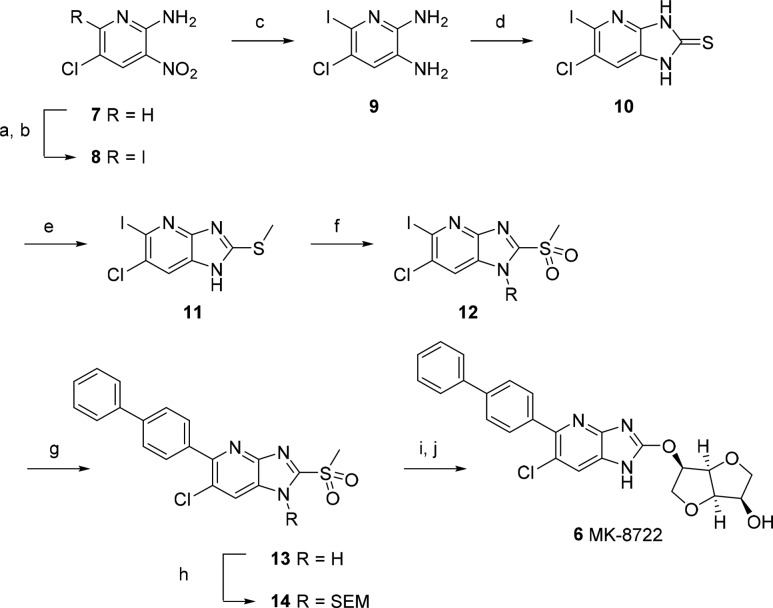
Scheme 1. Synthesis of MK-8722.
Reagents and conditions: (a) NCS, AcOH, 80 °C, 18 h; (b) NaI, AcOH, 90 °C, 2 h; (c) SnCl2·2H2O, EtOH, 70 °C, 0.5 h; (d) thiophosgene, DMAP, THF, 22 °C, 1 h; (e) KOH, MeI, EtOH, 22 °C, 0.5 h; (f) oxone, MeCN/H2O, 22 °C, 18 h; (g) 4-biphenylboronic acid, K3PO4, Pd(OAc)2, n-butyldiadamantylphosphine, THF/H2O, 45 °C, 18 h; (h) Et3N, SEMCl, THF, 0–22 °C, 15 min; (i) 1,4:3,6-dianhydro-2-O-[tert-butyl(dimethyl)silyl]-d-mannitol, DBU, DMA, 22 °C, 18 h; (j) KHSO4, HCO2H, 22 °C, 18 h then NaOH, THF, 0–22 °C, 18 h.