Abstract
Schizophrenia (SZ) is associated with increased somatic morbidity and mortality, in addition to cognitive impairments similar to those seen in normal aging, which may suggest that pathological accelerated aging occurs in SZ. Therefore, we aim to evaluate the relationships of age, telomere length (TL), and CCL11 (aging and inflammatory biomarkers, respectively), gray matter (GM) volume and episodic memory performance in individuals with SZ compared to healthy controls (HC). One hundred twelve participants (48 SZ and 64 HC) underwent clinical and memory assessments, structural MRI, and had their peripheral blood drawn for biomarkers analysis. Comparisons of group means and correlations were performed. Participants with SZ had decreased TL and GM volume, increased CCL11, and worse memory performance compared to HC. In SZ, shorter TL was related to increased CCL11, and both biomarkers were related to reduced GM volume, all of which were related to worse memory performance. Older age was only associated with reduced GM, but longer duration of illness was related with all the aforementioned variables. Younger age of disease onset was associated with increased CCL11 levels and worse memory performance. In HC, there were no significant correlations except between memory and GM. Our results are consistent with the hypothesis of accelerated aging in SZ. These results may indicate that it is not age itself, but the impact of the disease associated with a pathological accelerated aging that leads to impaired outcomes in SZ.
Keywords: schizophrenia, pathological accelerated aging, biomarkers, episodic memory, gray matter volume
Introduction
Schizophrenia (SZ) is associated with strong burden of disease1 and higher mortality, with a relative risk of all-cause mortality of 2.542 and an average life span of 15–25 years shorter than unaffected individuals.3 Compared to the general population, the median standardized mortality ratio in SZ is 2.41 if only natural causes of death are considered.4 This is related to the increased risk for several somatic diseases in SZ that are typically associated with the process of aging, such as cardiovascular disease, diabetes, and cancer.5 These observations of increased somatic morbidity and mortality, in addition to cognitive impairments similar to those seen in normal aging, may suggest that a “pathological accelerated aging” occurs in SZ.6 However, the neurobiological underpinnings and possible progression of SZ are still unclear, and studies are needed to generate evidence in regards to this theory.
A suggested biomarker of aging is telomere length (TL). Telomeres are DNA-protein structures that protect the ends of chromosomes and progressively shorten with each cell division.7 There is growing evidence linking TL with psychiatric disorders,8 especially showing that TL is shortened in SZ compared to healthy controls (HC).9,10 Shortened TL is related to more rapid cell senescence and apoptosis in association with aging and disease.11 More recently, TL was proposed to be a biomarker of “somatic redundancy”, which is the body’s capacity to absorb damage over time.12 TL is a heritable trait, but it is also influenced by epigenetic, environmental,8 inflammatory, and oxidative stress factors.13,14
Among inflammatory mediators that could be related to an accelerated aging process, SZ has been consistently associated with abnormalities in cytokines, which are proteins involved in the coordination of immune responses and exertion of neuromodulatory actions.15–19 A special type of cytokines that regulate the migration of peripheral immune cells directing them to pro-inflammatory activation states is the chemokines, which play a role in the central nervous system by regulating the inflammatory state associated with various pathological conditions.20 Besides their role in inducing and directing leukocyte migration, chemokines have been implicated in other neurobiological processes. For instance, the binding of a chemokine to its receptor activates signaling cascades that result in increased calcium concentrations and the activation of mitogen-activated protein kinases, important mechanisms for synaptic plasticity.21 Moreover, the pro-inflammatory chemokine CCL11 was demonstrated to be an age-related systemic factor associated with decreased neurogenesis in hippocampus and impaired learning and memory in mice.22 Previous studies showed that CCL11 was increased in chronic, though not in recent onset individuals with SZ, consistent with the hypothesis that it could also be a potential biomarker for a pathological aging process in SZ.23,24
In normal aging, there is progressive whole brain volume loss in late adulthood.25 In SZ, brain volumes are consistently decreased.26 However, a longitudinal study evaluating gray matter (GM) density maps showed that this whole brain volume reduction may be due to possible faster aging compared to HC, especially in the first years after disease onset. At baseline, brain age in SZ was 3.36 years greater than chronological age, and SZ showed a further accelerated aging of an additional 4 months in each year after follow-up. The authors proposed that 2 different processes might influence progressive brain loss in SZ: accelerated aging of the brain and other factors influencing individual variation, such as medication use.27 Another study revealed a diagnosis by age interaction in the prediction of efficiency of the cingulo-opercular and fronto-parietal networks,28 which are associated with cognitive ability in both health and SZ.29
Performance across several cognitive domains is also decreased in SZ,30 even in drug-naïve patients.31 However, it is not clear whether cognitive impairments follow a normal rate of age-related changes or also present with faster aging. One meta-analysis showed similar changes in cognitive performance in later life compared to aging in general population, although patients had worst functioning.32 Individuals with SZ show an early cognitive impairment, what seems to be related to neurodevelopmental abnormalities.33 After the disease onset, there is evidence for stability of cognitive functioning over time.34 However, individuals with SZ over 65–70 years old showed relatively greater age associated differences in cognitive functioning than healthy individuals,35,36 which may indicate a link between abnormal neurodevelopmental and neurodegenerative process in SZ.37 Episodic memory impairments show a particularly large effect size in SZ,38 and have been associated with daily functioning.39,40 Interestingly, a recent study showed that memory impairment in SZ was similar to the impairment seen in healthy aging, possibly pointing to shared mechanisms.41 Memory performance clearly reduces with aging, with a particularly strong effect on the ability to learn new associations.42 Accordingly, episodic memory may be sensitive to the effects of both normal and pathological aging,43 and may be a useful behavioral indicator of biological aging related outcome. Also, it would be important to understand the possible mechanisms related to memory deficits in SZ, although the present study is not limited to the analysis of cognitive aging.
However, it is unclear whether the pathological aging hypothesis could reflect the number of years that the individual has been alive, or the duration of the person’s illness, and whether these variables relate differently to the above mentioned possible aging markers. If the abnormalities seen in SZ are explained only by age, this could mean a process that parallels that seen in aging among individuals who do not have SZ. However, if the abnormalities in SZ are better explained by illness duration, this could represent a process more specific to serious mental illness such as SZ, in which the burden of the disease leads to impairments.
Given the evidence outlined above, the current study was designed to investigate the following questions: (1) are aging and inflammatory biomarkers more strongly related in SZ than HC?; (2) are aging and inflammatory biomarkers more strongly related to GM volume in SZ than HC?; (3) are aging and inflammatory biomarkers and GM volume more strongly related to memory performance in SZ than HC?; (4) is age related differently in SZ than HC to aging and inflammatory biomarkers, GM volume and memory?; (5) in SZ, are illness duration, age of disease onset and current psychopathological state related to aging and inflammatory biomarkers, GM volume and memory?; and (6) in SZ, are there any indirect effects of aging and inflammatory biomarkers or GM volume?
Methods
Participants
There were 112 participants: 48 individuals with SZ and 64 unaffected individuals (HC). All participants were between the ages of 18 and 60 years, and were recruited from the same general public hospital in Brazil. The SZ and HC subjects were similar in age, sex, and education and all originated from the same socioeconomic and educational background. The Institutional Review Board approved the study protocol. All participants were advised about the procedures and signed informed consent prior to participation.
SZ had their diagnoses confirmed by trained psychiatrists using the Structured Clinical Interview for DSM-IV (SCID). Their psychopathological state was assessed using the 18-item Brief Psychiatry Rating Scale (BPRS).44 They had to be stable for at least 6 months and could not be currently in a psychotic episode. HC had no current or previous history, nor first-degree family history of a major psychiatric disorder, including dementia or intellectual disability, confirmed by the SCID.
Additional exclusion criteria for the groups were history or presence of neurological disease, abuse or dependence on drugs, brain tumor, thyroid disease, rheumatological disease, uncontrolled endocrine and cardiac disease, history of autoimmune diseases or chronic infections/inflammatory diseases, having any severe systemic disease, or having received immunosuppressive therapy.
Clinical and Memory Assessment
Participants underwent clinical evaluation with trained psychiatrists to collect sociodemographic, clinical and pharmacological data through a structured interview. Furthermore, patient’s hospital clinical records were searched to collect supplementary data. All participants were assessed by trained psychologists with the Hopkins Verbal Learning Test-Revised (HVLT-R), which is a word-list task widely used to measure verbal learning and episodic memory.45 The HVLT-R is part of the MATRICS Consensus Battery for Schizophrenia.46 It is comprised of 3 immediate recall trials of 12 words within 3 categories and a delayed recall followed by a recognition task. We focused our analyses on total immediate recall.
Biomarkers
The complete description is in the supplementary methods and it was described elsewhere.10,47
Neuroimaging
T1-weighted magnetic resonance images were acquired with a Philips Achieva 1.5 T scanner. Volumetric segmentations were performed using the Freesurfer image analysis suite software v.5.1.0 (http://surfer.nmr.mgh.harvard.edu/). Technical details are described elsewhere.48–50 All images were processed and checked by the same researcher. Our analyses focused on total GM volume (cortical + subcortical).
Data Analysis
Statistical analyses were performed in R (https://www.R-project.org/). We first compared groups by examining demographic, clinical, memory performance, aging and inflammatory biomarkers and GM volume variables using Student’s t test or chi-square test. For the total GM volume, intracranial volume was regressed out. Our second level of analysis tested our hypotheses about the relationships between variables within each group using Pearson’s correlation coefficient, and Fisher’s z transformation to compare the magnitude of correlations across groups. We chose to statistically compare the magnitude of correlations across groups to simplify the interpretation of the findings, but regression models with interaction terms between diagnostic group and predictor variables provided the same results as described below (supplementary material). Lastly, we wanted to suggest possible pathways of these relationships. Therefore, we conducted a mediation model using the PROCESS model with aging and inflammatory biomarkers as dependent variables predicting memory performance with total GM residual volume as the mediator.
Results
Diagnostic Group Comparisons
HC and SZ (table 1) had similar age, gender distribution, education, and Body Mass Index. SZ had more tobacco use than HC. As expected, SZ had worse episodic memory performance compared to HC. Also as predicted, SZ had increased CCL11 levels and reduced TL compared to HC. After we regressed out intracranial volume from total GM volume, SZ showed decreased residuals of total GM volume.
Table 1.
Diagnostic Group Comparisons
| Variables, Mean (SD) | Healthy Controls, n = 60 | Individuals With Schizophrenia, n = 48 | t test/ Chi-Square |
|---|---|---|---|
| Age (y) | 36.40 (12.96) | 35.12 (12.05) | t (106) = .524, P = .601 |
| Gender (male/female) | 36/24 | 33/15 | X 2 = .546, df = 1, P = .459 |
| Education (y) | 11.05 (3.22) | 10.31 (3.17) | t (106) = 1.19, P = .237 |
| BMI | 26.08 (4.40) | 25.96 (5.11) | t (90) = .126, P = .900 |
| Tobacco use (n/day) | 1.68 (5.26) | 10.46 (15.11) | t (102) = −4.073, P < .001* |
| Age of onset (y) | — | 22.45 (6.10) | — |
| Illness duration (y) | — | 12.74 (11.60) | — |
| BPRS | — | 14.88 (12.56) | — |
| Medication (%) | |||
| Typical antipsychotics | — | 15% | — |
| Atypical antipsychotics | — | 27% | — |
| Clozapine | — | 58% | — |
| HVLT-R total immediate recall | 24.53 (4.53) | 17.12 (5.61) | t (99) = 7.326, P < .001* |
| CCL11 levels | 815.51 (592.77) | 1531.18 (1251.87) | t (82) = −3.496, P < .001* |
| Telomere length | 1.36 (0.65) | 0.95 (0.41) | t (79) = 3.345, P = .001* |
| Total gray matter residual volumea | 21688.50 (28445.65) | −19137.55 (43403.48) | t (101) = 5.75, P < .001* |
Note: BMI, body mass index; BPRS, Brief Psychiatric Rating Scale; HVLT-R, Hopkins Verbal Learning Test—Revised.
aIntracranial volume was regressed out.
*Statistically significant.
All correlational analyses are described in supplementary table 1.
Are Aging and Inflammatory Biomarkers Related?
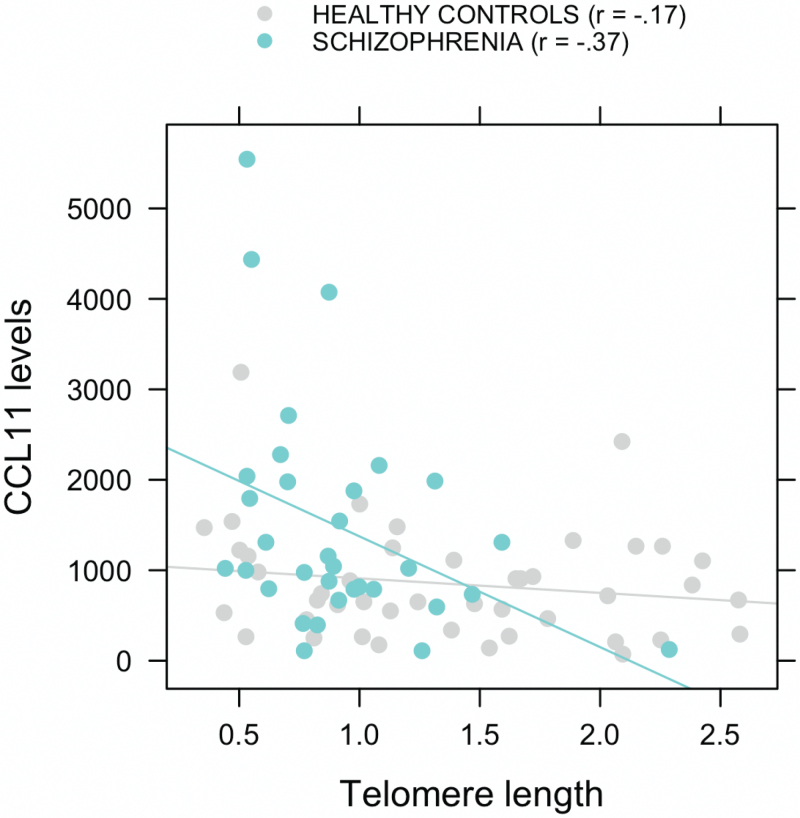
As shown in figure 1, TL and CCL11 were significantly negatively correlated in SZ (r = −.37, P = .032). In HC, this correlation was not significant (r = −.17, P = .27), although its magnitude was not statistically different than SZ (z = −0.90, P = .37).
Fig. 1.
Correlation between CCL11 levels and telomere length in subjects with schizophrenia and healthy controls.
Are Aging and Inflammatory Biomarkers Related to GM Volume?
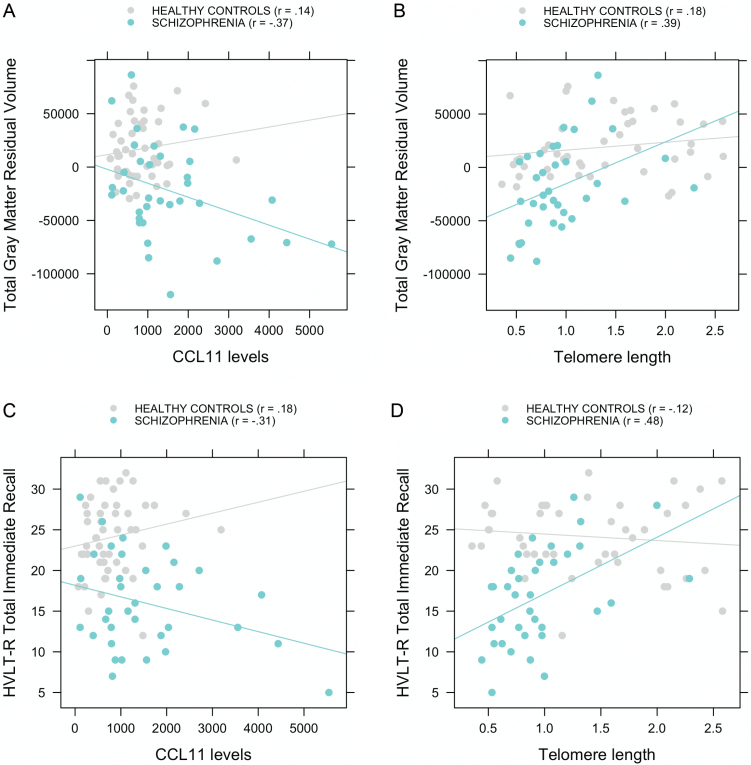
CCL11 and total GM volume were significantly negatively correlated in SZ (r = −.37, P = .03), but not in HC (r = .14, P = .35), and these relations were statistically different between groups (z = −2.27, P = .02; figure 2A). TL and total GM volume were positively correlated in SZ (r = .39, P = .02). In HC, although the correlation was not statistically significant (r = .18, P = .25), the Fisher transformation showed no significant difference between groups in the magnitude of the correlation (z = 0.98, P = .33; figure 2B).
Fig. 2.
Correlations between CCL11 levels and telomere length to gray matter volume after regressing out intracranial volume (A and B) and to episodic memory performance (C and D) in subjects with schizophrenia and healthy controls.
Are Aging and Inflammatory Biomarkers and GM Volume Related to Memory Performance?
CCL11 and HVLT-R total immediate recall were negatively correlated in SZ at a trend level (r = −.31, P = .07), but not in HC (r = .18, P = .23), and these relations were statistically different between groups (z = −2.17, P = .03; figure 2C). TL and HVLT-R total immediate recall were positively correlated in SZ (r = .48, P = .003), but not in HC (r = −.12, P = .45), and these relations were also significantly different (z = 2.75, P = .006; figure 2D). In both SZ and HC, total GM volume and HVLT-R total immediate recall were positively correlated (r = .41, P = .005 and r = .27, P = .05, respectively).
What is the Relationship of Age to Biomarkers, GM Volume, and Memory?
TL and CCL11 were not significantly correlated with age in either SZ (r = −.30, P = .078 and r = .29, P = .092, respectively) or HC (r = −.10, P = .51 and r = −.05, P = .72, respectively). Total GM volume and age were negatively correlated in both SZ (r = −.65, P < .001) and HC (r = −.71, P < .001). Age and HVLT-R total immediate recall were not significantly correlated in SZ (r = −.23, P = .122), but were negatively correlated in HC (r = −.40, P = .003), although the magnitude of this correlation was not statistically different than SZ (z = 0.98, P = .33).
What is the Relationship of Clinical Characteristics of SZ to Biomarkers, GM Volume and Memory?
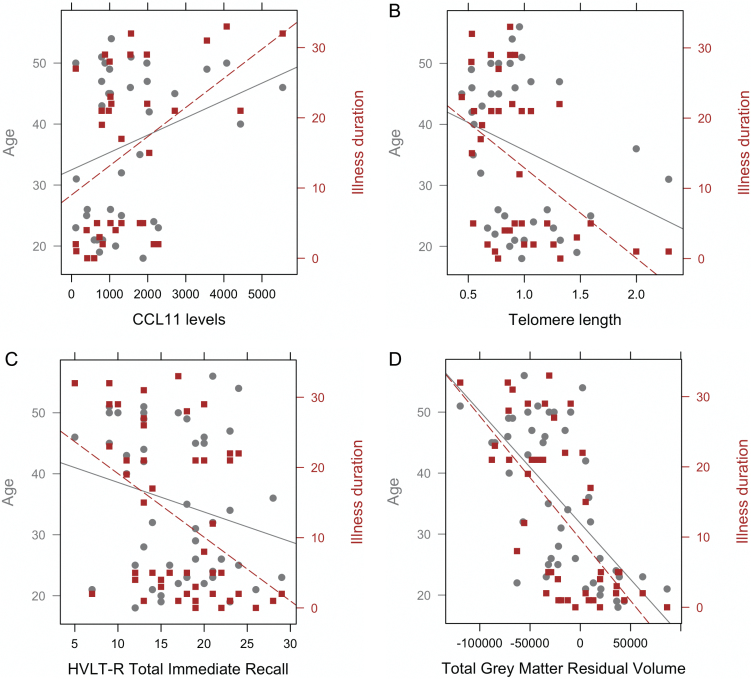
In SZ, illness duration was significantly correlated with TL (r = −.46, P = .004), CCL11 (r = .45, P = .007) total GM volume (r = −.66, P < .001) and HVLT-R total immediate recall (r = −.44, P = .002). Age of disease onset was only correlated with CCL11 (r = −.34, P = .043) and HVLT-R total immediate recall (r = .39, P = .006). BPRS total score was not significantly correlated with any of the variables (P > .29). We used the method of Meng, Rosenthal and Rubin51 for comparing correlated correlation coefficients to compare the magnitude of the correlations with age vs illness duration. Not surprisingly, age and illness duration were strongly correlated (r = .87, P < .001). However, illness duration was significantly more strongly correlated than age with TL (Z = 2.29, P = .01), CCL11 (Z = −0.28, P = .01), and HVLT-R (Z = 2.95, P = .002), but not for GM volume (Z = 0.18, P = .42; figure 3).
Fig. 3.
Correlations between age (left y-axis) and illness duration (right y-axis) to: (A) CCL11 levels (r = .29 and r = .45, respectively), (B) telomere length (r = −.30 and r = −.46, respectively), (C) episodic memory performance (r = −.23 and r = −.44, respectively) and (D) gray matter volume after regressing out intracranial volume (r = −.65 and and r = −.66, respectively) in subjects with schizophrenia.
Does GM Volume Mediate the Relationship Between Aging and Inflammatory Biomarkers and Memory in SZ?
We created 2 different mediation models. First, we included TL as the independent variable, GM volume as the mediator, and HVLT-R total immediate recall as the dependent variable. There was no significant indirect effect of GM volume on HVLT-R total immediate recall (P = .23). We created a second model with CCL11 as the independent variable, GM volume as the mediator, and HVLT-R total immediate recall as the dependent variable. There was a trend indirect effect of GM volume predicting HVLT-R total immediate recall (P = .04). The bootstrapped unstandardized indirect effect was −0.0006, and the 95% CI ranged from −0.0014, 0.0001, suggesting that CCL11 may predict memory performance in part through GM volume.
Does Aging and Inflammatory Biomarkers or GM Volume Mediate the Relationship Between Illness Duration and Memory in SZ?
We included illness duration as the independent variable, HVLT-R total immediate recall as the dependent variable and CLL11, TL, and GM volume as separate mediators. There were no significant indirect effects of either CCL11 or GM volume on HVLT-R (P = .29 and P = .14, respectively). However, we found a significant indirect effect of TL on HVLT-R total immediate recall (P = .02). The bootstrapped unstandardized indirect effect was −.10, and the 95% CI ranged from −.23, −.03. Therefore, TL mediated the effect of illness duration to memory performance.
Discussion
This study is, to the best of our knowledge, the first to show associations between TL, CCL11 levels, GM volume, and episodic memory performance in people with SZ, presenting new evidence relevant to the theory of pathological accelerated aging in SZ. As expected, compared to demographically similar HC, individuals with SZ had worse memory performance, increased CCL11, reduced TL, and decreased residuals of total GM volume after regressing out intracranial volume. In SZ, shorter TL was related with increased CCL11 levels, and they were both related to reduced GM volume. Shorter TL, increased CCL11 levels and reduced GM volume were all related to worse memory performance. Compared to HC, SZ showed statistically stronger magnitudes of correlations between shorter TL and increased CCL11 levels, and between shorter TL and reduced GM volume. Among SZ, age was significantly correlated only with reduced GM volume. Longer duration of illness was related with all the aforementioned variables. The effects of duration of illness to memory performance were mediated by TL. Younger age of disease onset was related with increased CCL11 levels and worse memory performance. These results may indicate that it is not age itself, but the impact of the disease associated with a pathological aging that leads to worse outcomes among SZ. Each of these sets of findings will be discussed in more detail below.
First, we saw that the aging and inflammatory biomarkers TL and CCL11 were significantly more strongly related in SZ compared to HC. There are several theories that have been proposed to explain normal aging, which is a complex process determined by multiple factors that include genetic, environmental and socioeconomic influences. Aging can be defined as a collection of time-dependent anatomical and physiological changes that reduce functional capacity, physiological and homeostatic reserve and decrease the ability to adjust to stress.52 There is consistent evidence that associates TL with aging and mortality, although these relationships are not clearly causational.53 Telomeres are implicated in cellular aging and human diseases of premature aging, but it is not a unique and universal aging biomarker.54 Diseases of short TL are known to represent premature aging, as they show processes that occur in subjects as they age, including features marked by vascular and degenerative components, as well as by cancer predisposition.7 Hence, TL could be an integrative measure of somatic damage or history of past cell replication,53 representing the pace of biological aging related to the lifespan of cells and the body. The shortening of TL may be accelerated by the cumulative impact of stressors, which in turn may speed the process of biological aging.55 We saw that SZ had shorter TL compared to HC, what could mean that these individuals had greater impairment related to a faster somatic aging and/or increased damage. Interestingly, TL was significantly correlated to duration of illness and not to age, which is consistent with the hypothesis of a greater impact of accumulated stress or adversity. Furthermore, TL mediated the effect of illness duration to memory performance, suggesting important functional implications of these processes.
In SZ, shorter TL was associated with increased CCL11 levels, which were significantly increased compared to HC. CCL11 is a chemokine also associated with older age.22 During inflammatory response and oxidative stress, there is an increased immune cell replication that potentially affects TL.8 Thus in SZ, CCL11 could be involved in a pathogenic pathway of oxidative stress/pro-inflammatory imbalance that could lead to accelerated cell aging.3 People with SZ often present a multi-morbidity state, ie, the presence of 2 or more chronic conditions in the same individual, which is associated with increased inflammation that contributes to accumulation of a disease burden.56 As such, the relationship between TL and CCL11 is consistent with the idea that TL may be a biomarker of body’s capacity to absorb damage over time,12 with shorter TL reflecting less capacity to do so.
Both shorter TL and increased CCL11 levels were related to reduced total GM volume in SZ, but not HC, and the relationship between CCL11 and GM was statistically stronger in SZ than HC. These relationships are consistent with the idea that chronic pro-inflammatory processes could influence the brain through mechanisms such as neuroinflammation and microglial activation, which in turn could lead to structural and functional consequences, such as loss of GM.57 Alterations in immune function early in life may lead to increased inflammation over time, which in turn can produce brain abnormalities.58 Interestingly, a recent diffusion tensor imaging (DTI) study showed an age-by-diagnoses interaction for global fractional anisotropy (FA), which suggested faster decline of cerebral white matter (WM) in patients with SZ compared with controls.59 Such result could be explained by inflammatory processes, a mechanism that could also be contributing to the reduced GM volume detected in the current study. Interestingly, increased levels of CCL11 were significantly related to longer duration of illness, which in turn was related to reduced GM volumes and worse memory performance in SZ. Additionally, increased CCL11 levels were related to younger age of disease onset. These observations could fit with the neurodevelopmental hypothesis of SZ.60 There is considerable evidence of pathological risk factors such as early-life infections that influence early neurodevelopment in SZ61 and affect the brain before it approaches its adult anatomical state.60 The increased risk of developing SZ associated with the history of varying types of infections may suggest that a pro-inflammatory immune response may contribute to the onset of SZ.62 Recently, the North American Prodrome Longitudinal Study (NAPLS) identified markers of inflammation, oxidative stress, and dysregulation of hypothalamic-pituitary axis that were able to predict the conversion from clinical high risk to psychosis.63 Thus, the neurodevelopmental course of SZ might trigger or be linked to chronic inflammation that in turn may be associated with the onset of the disease and a worse outcome as the illness progresses, producing an accelerated pathological aging.
In addition, aging and inflammatory biomarkers were more strongly related to memory performance in SZ than HC. There is evidence that memory performance shows age-related impairments43 and vulnerability to different biological processes affecting the brain in illness and healthy aging.37 Worse memory performance in SZ was associated with increased CCL11, and this relationship was significantly stronger than the relationship in HC. This result is consistent with other studies linking inflammation to cognitive deficits in SZ, although the mechanisms of this relationship are still unclear.64 One possible explanation would be the aforementioned processes of chronic inflammation influencing the brain.57 Interestingly, even in our relatively small sample size, we found a small trend effect of GM volume mediating the effect of CCL11 on memory performance, which points toward a possible mechanism to be further investigated.
Worse memory performance was associated with shorter TL in SZ but not in HC. However, better memory performance was correlated with larger GM volume in both groups, even though individuals with SZ had significantly worse scores on HVLT-R. From the mediation model, we saw that TL predicted memory performance not through an indirect effect of GM volume. These results suggest that memory impairment in SZ might have multiple contributing factors, including the impact of stress and other factors associated with pathological aging,12 as indexed by TL and CCL11 levels, though only the latter may be partially mediated by GM reductions.
Lastly, age and illness duration were differently related to aging and inflammatory biomarkers and memory performance in SZ. In SZ, age was only correlated with GM, but duration of illness was associated with TL, CCL11, GM, and memory performance, with significantly stronger correlations to all but GM. One might have expected stronger relationships to age given the accelerated aging hypothesis. However, the stronger correlations with illness duration suggest that it is not age itself, but rather the impact of the disease that relates to these impairments. These findings may be moderated or mediated by factors such as medication and lifestyle that themselves may influence biological mechanisms of aging in SZ. In HC, however, older age was related with worse memory performance in addition to reduced GM volume, which goes in line with what is described in the literature regarding normal aging. It should be mentioned that an accelerated aging process seems to be a particular finding of SZ, and it is unclear whether other mental illnesses, such as major depression and bipolar disorder, have similar trajectories.65,66
The findings of the present article highlight the importance of considering the course of SZ in a broader perspective, one that focus on reducing the burden of the disease during its trajectory, which could in turn decrease the impairments associated with a pathological aging. This perspective emphasizes the idea that patients should receive effective treatments that include not only psychotropic drugs, but more comprehensive approaches to improving health and well-being, such as diet, physical exercise, and health care. Further, these findings suggest that the clinical evaluation of age related cognitive declines may need to start in an earlier age among individuals with psychosis.
Our study had limitations. It was a cross sectional design with a relatively limited sample size, which restrict the conclusions that can be made from the results. Cross sectional studies are useful to generate hypotheses regarding aging, but they do not allow for individuals to be followed along their aging process and therefore make it difficult to separate the effects of age from other influences or to understanding temporal dynamics within individuals that may inform causal mechanisms. To fully understand aging mechanisms and trajectories of SZ, longitudinal studies will be necessary. Further, there are likely confounds that are associated with SZ and that could influence the analysis of aging and inflammatory biomarkers, such as comorbidities, lifestyle factors or medication effects, which were not able to be addressed in this study. We did not include in the analysis the effect of current medication or history of psychotropic treatment, although all individuals with SZ were stable for at least 6 months. Also, we only presented results of analyses of brain structure, and we did not have data on brain function or connectivity among these individuals, which may also show important relationships to cognitive function. Finally, we did not have data on the premorbid intellectual functioning, what could have influenced memory performance.
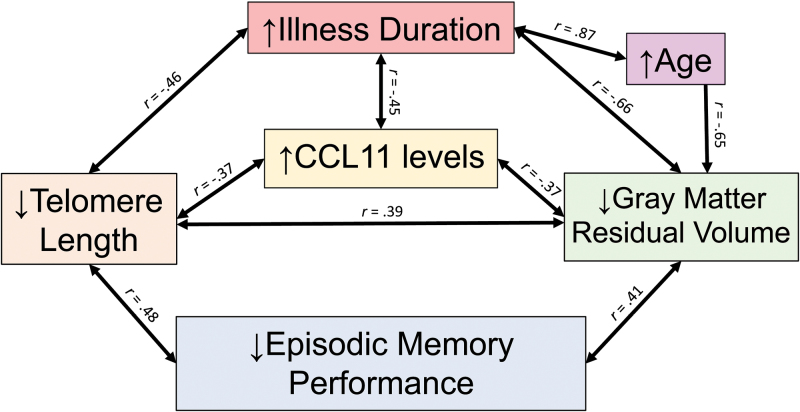
In summary, our results are consistent with the hypothesis of pathological accelerated aging in SZ. We saw associations between increased CCL11—an age-related pro-inflammatory marker, shorter TL, reduced GM volume, and decreased episodic memory in SZ, which were all related to longer duration of illness (figure 4). These results suggest that it is not age itself, but the impact of the disease associated with a pathological aging that might lead to a worse outcome. Although preliminary, our data point to potentially important mechanisms related to neural and cognitive impairment associated with psychosis. Future steps should focus on longitudinal studies of individuals at high risk to develop psychosis to evaluate the effect of inflammatory and aging biomarkers in the conversion, but also should follow individuals with SZ across their lifespan to confirm the accelerated aging hypothesis.
Fig. 4.
Possible mechanisms involved in a pathological accelerated aging of schizophrenia, based on the relationships observed in this study.
Supplementary Material
Supplementary data are found at Schizophrenia Bulletin online.
Funding
This study was supported by CNPq, CAPES and FIPE/HCPA, Brazil. It received grants from CNPq (Universal 443526/2014-1, Universal 458294/2014-4, PQ 304443/2014-0).
Supplementary Material
Acknowledgments
The authors also thank Fátima Theresinha Costa Rodrigues Guma for the support provided. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. [DOI] [PubMed] [Google Scholar]
- 2. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time?Arch Gen Psychiatry. 2007;64:1123–1131. [DOI] [PubMed] [Google Scholar]
- 5. Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452–464. [DOI] [PubMed] [Google Scholar]
- 6. Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging?Schizophr Bull. 2008;34:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindqvist D, Epel ES, Mellon SH, et al. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polho GB, De-Paula VJ, Cardillo G, dos Santos B, Kerr DS. Leukocyte telomere length in patients with schizophrenia: a meta-analysis. Schizophr Res. 2015;165:195–200. [DOI] [PubMed] [Google Scholar]
- 10. Czepielewski LS, Massuda R, Panizzutti B, et al. Telomere length in subjects with schizophrenia, their unaffected siblings and healthy controls: evidence of accelerated aging. Schizophr Res. 2016;174:39–42. [DOI] [PubMed] [Google Scholar]
- 11. Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest. 2013;123:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boonekamp JJ, Simons MJ, Hemerik L, Verhulst S. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell. 2013;12:330–332. [DOI] [PubMed] [Google Scholar]
- 13. Baylis D, Ntani G, Edwards MH, et al. Inflammation, telomere length, and grip strength: a 10-year longitudinal study. Calcif Tissue Int. 2014;95:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. [DOI] [PubMed] [Google Scholar]
- 15. Monji A, Kato T, Kanba S. Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–265. [DOI] [PubMed] [Google Scholar]
- 16. Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64:217–230. [DOI] [PubMed] [Google Scholar]
- 17. Domenici E, Willé DR, Tozzi F, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One. 2010;5:e9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–286. [DOI] [PubMed] [Google Scholar]
- 19. Pedrini M, Massuda R, Fries GR, et al. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res. 2012;46:819–824. [DOI] [PubMed] [Google Scholar]
- 20. Stuart MJ, Singhal G, Baune BT. Systematic review of the neurobiological relevance of chemokines to psychiatric disorders. Front Cell Neurosci. 2015;9:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rostène W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator?Nat Rev Neurosci. 2007;8:895–903. [DOI] [PubMed] [Google Scholar]
- 22. Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teixeira AL, Reis HJ, Nicolato R, et al. Increased serum levels of CCL11/eotaxin in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:710–714. [DOI] [PubMed] [Google Scholar]
- 24. Pedrini M, Massuda R, de Lucena D, et al. Differences in eotaxin serum levels patients with recent onset and in chronic stable schizophrenia: a clue for understanding accelerating aging profile. Schizophr Res. 2014;152:528–529. [DOI] [PubMed] [Google Scholar]
- 25. Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33:1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schnack HG, van Haren NE, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated brain aging in schizophrenia: a longitudinal pattern recognition study. Am J Psychiatry. 2016;173:607–616. [DOI] [PubMed] [Google Scholar]
- 28. Sheffield JM, Repovs G, Harms MP, et al. Evidence for accelerated decline of functional brain network efficiency in schizophrenia. Schizophr Bull. 2016;42:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheffield JM, Repovs G, Harms MP, et al. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res. 2014;158:156–162. [DOI] [PubMed] [Google Scholar]
- 32. Irani F, Kalkstein S, Moberg EA, Moberg PJ. Neuropsychological performance in older patients with schizophrenia: a meta-analysis of cross-sectional and longitudinal studies. Schizophr Bull. 2011;37:1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meier MH, Caspi A, Reichenberg A, et al. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry. 2014;171:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bergh S, Hjorthøj C, Sørensen HJ, et al. Predictors and longitudinal course of cognitive functioning in schizophrenia spectrum disorders, 10 years after baseline: The OPUS study. Schizophr Res. 2016;175:57–63. [DOI] [PubMed] [Google Scholar]
- 35. Loewenstein DA, Czaja SJ, Bowie CR, Harvey PD. Age-associated differences in cognitive performance in older patients with schizophrenia: a comparison with healthy older adults. Am J Geriatr Psychiatry. 2012;20:29–40. [DOI] [PubMed] [Google Scholar]
- 36. Harvey PD. What is the evidence for changes in cognition and functioning over the lifespan in patients with schizophrenia?J Clin Psychiatry. 2014;75(suppl 2):34–38. [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi H, Isohanni M, Jääskeläinen E, et al. Linking the developmental and degenerative theories of schizophrenia: association between infant development and adult cognitive decline. Schizophr Bull. 2014;40:1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia?Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 40. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”?Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 41. Silver H, Bilker WB. Similar verbal memory impairments in schizophrenia and healthy aging. Implications for understanding of neural mechanisms. Psychiatry Res. 2015;226:277–283. [DOI] [PubMed] [Google Scholar]
- 42. Silver H, Goodman C, Bilker WB. Impairment in associative memory in healthy aging is distinct from that in other types of episodic memory. Psychiatry Res. 2012;197:135–139. [DOI] [PubMed] [Google Scholar]
- 43. Tromp D, Dufour A, Lithfous S, Pebayle T, Després O. Episodic memory in normal aging and Alzheimer disease: insights from imaging and behavioral studies. Ageing Res Rev. 2015;24:232–262. [DOI] [PubMed] [Google Scholar]
- 44. Romano F, Elkis H, Tradução e adaptação de um instrumento de avaliação psicopatológica das psicoses: a Escala Breve de Avaliação Psiquiátrica - Versão Ancorada (BPRS-A). J Bras Psiquiatr. 1996;45:43–49. [Google Scholar]
- 45. Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 46. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 47. Panizzutti B, Gubert C, Schuh AL, et al. Increased serum levels of eotaxin/CCL11 in late-stage patients with bipolar disorder: an accelerated aging biomarker?J Affect Disord. 2015;182:64–69. [DOI] [PubMed] [Google Scholar]
- 48. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 49. Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vianna-Sulzbach M, Rocha NP, Teixeira AL, et al. Right hippocampus size is negatively correlated with leptin serum levels in bipolar disorder. Psychiatry Res. 2015;230:719–721. [DOI] [PubMed] [Google Scholar]
- 51. Meng X, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychol Bull. 1992;111:172–175. [Google Scholar]
- 52. Ahmed A, Tollefsbol T. Telomeres and telomerase: basic science implications for aging. J Am Geriatr Soc. 2001;49:1105–1109. [DOI] [PubMed] [Google Scholar]
- 53. Simons MJ. Questioning causal involvement of telomeres in aging. Ageing Res Rev. 2015;24:191–196. [DOI] [PubMed] [Google Scholar]
- 54. Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66:202–213. [DOI] [PubMed] [Google Scholar]
- 55. Reynolds CF., III Telomere attrition: a window into common mental disorders and cellular aging. Am J Psychiatry. 2016;173:556–558. [DOI] [PubMed] [Google Scholar]
- 56. Stepanova M, Rodriguez E, Birerdinc A, Baranova A. Age-independent rise of inflammatory scores may contribute to accelerated aging in multi-morbidity. Oncotarget. 2015;6:1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leza JC, García-Bueno B, Bioque M, et al. Inflammation in schizophrenia: a question of balance. Neurosci Biobehav Rev. 2015;55:612–626. [DOI] [PubMed] [Google Scholar]
- 58. Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kochunov P, Ganjgahi H, Winkler A, et al. Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp. 2016;37:4673–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Perkins DO, Jeffries CD, Addington J, et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull. 2015;41:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ribeiro-Santos A, Lucio Teixeira A, Salgado JV. Evidence for an immune role on cognition in schizophrenia: a systematic review. Curr Neuropharmacol. 2014;12:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kochunov P, Glahn DC, Rowland LM, et al. Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biol Psychiatry. 2013;73:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koutsouleris N, Davatzikos C, Borgwardt S, et al. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull. 2014;40:1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.