Figure 1.
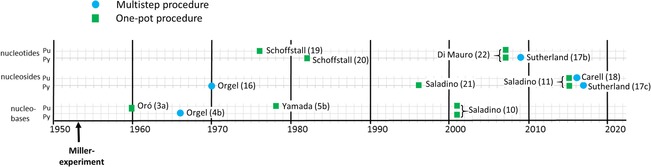
Chronology of selected historically innovative one‐pot and multistep syntheses of nucleic acid building blocks. Oró (1960):3a ammonium hydroxide, HCN, H2O at 90 °C. Orgel (1966):4 step 1: formamidine, NH2CH(CN)2, EtOH at reflux; step 2: imidazole intermediate, formamidine, methyl cellosolve at reflux. Orgel (1970):16 step 1: sugar monophosphate, NCNH2, H2O; step 2: oxazoline intermediate, N≡CC≡CH; step 3: photochemistry. Schoffstall (1976):19 purine nucleoside, KH2PO4, formamide at 70 °C. Yamada (1978):5b formamide, HCN, sealed vial at 160 °C. Schoffstall (1982):20 pyrimidine nucleoside, KH2PO4, formamide at 125 °C. Saladino (1996):21 formamide at 160 °C. Saladino (2001):10 formamide, minerals at 160 °C. Di Mauro (2007):22 nucleoside, formamide, KH2PO4 or mineral phosphates at 90 °C; Sutherland (2009):17b step 1: glycolaldehyde, NCNH2, H2O; step 2: 2‐aminooxazole intermediate, glyceraldehyde, phosphate buffer; step 3: pentose aminooxazoline intermediate, N≡CC≡CH, phosphate buffer; step 4: anhydroarabinonucleoside intermediate, phosphate buffer. Saladino (2015):11 formamide, minerals, proton beam at 25 °C; Carell (2016):18 step 1: guanidine and aminocyanoacetamide; step 2: aminopyrimidinone intermediate, formic acid or formamide at reflux; step 3: formamide pyrimidinone intermediate, ribose, dry state followed by treatment with borax at 100 °C or NH3, or other conditions. Sutherland (2017):17c step 1: 2‐aminooxazole intermediate, and glyceraldehyde; step 2: pentose aminooxazoline intermediate, hydrosulfide hydrate, sodium hydrosulfide hydrate in formamide at 50 °C for 7 h; step 3: 2‐thioribocytidine intermediate, hydrosulfide hydrate, degassed water, irradiation at 254 nm for 2.5 days; step 4: NH4H2PO4, urea in formamide at 100 °C for 6 days. Pu: purines, Py: pyrimidines.
