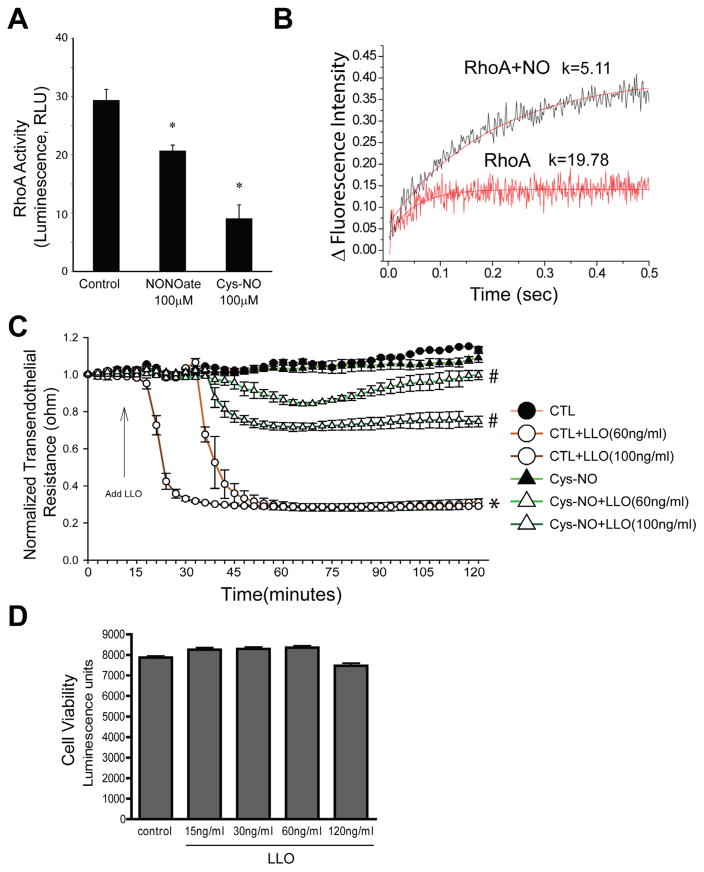
Fig. 2.
Nitric oxide is an inhibitor of RhoA activity and G+-toxin-induced microvascular permeability. (A) HLMVEC cells were grown to confluency in 100-mm dishes, treated with NONOate (100 μM) or Cys-NO (100 μM) for 30 min. Cells were then lysed and RhoA activity was determined by using the G-LISA RhoA activation assay. Data are expressed as means ± S.E., *P < 0.05 versus control. (n = 4–6). (B) The kinetics of binding fluorescently labeled GTP to RhoA was performed using stopped flow analysis. Recombinant RhoA (0.1 μM) was mixed with 2 μM of mant-GTP, excitation set to 350 nm and the cutoff filter for emission was 395 nm. Increased fluorescence upon binding mant-GTP was monitored over a 0.5 s time scale. RhoA nitrosylation was achieved by incubating with 100 μM MAHMA NONOate for 30 min prior to mixing with mant-GTP. Three measurements were averaged and fitted with bimolecular reaction equation. Nitric oxide decreased the binding constant of GTP to RhoA from 19.78 to 5.11. (C) HLMVECs were treated with or without Cys-NO (100 μM) for 30 min and then challenged with LLO (60 ng/ml and 120 ng/ml). Transendothelial resistance (TER) was monitored overtime using ECIS. Resistance was normalized to time = 0 and plotted as a function of time. Data are expressed as means ± S.E., *P < 0.05 versus control. #P < 0.05 versus LLO (n = 3–6). (D) LLO does not influence cell viability. Confluent HLMVECs were treated with LLO (0–120 ng/ml) for 1 h, and cell viability was measured using the CellTiter-Glo® Luminescent Cell Viability Assay. Data are expressed as means ± S.E. (n = 3–6).