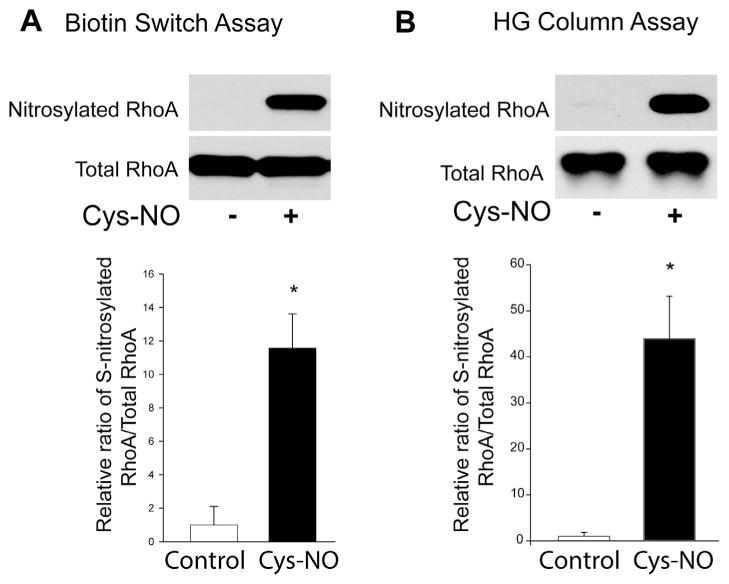
Fig. 3.
RhoA is a substrate for S-nitrosylation. (A) HLMVECs were treated with either vehicle or Cys-NO (100 μM) for 30 min, and the S-nitrosylation of proteins was determined by the biotin-switch assay in the presence of ascorbate and trace levels of copper. Biotinylated proteins were concentrated using streptavidin–agarose beads, and immunoblotted for RhoA (SNO-RhoA, top panel) versus total RhoA in cell lysates (total RhoA, bottom panel). (B) HLMVECs were treated with or without Cys-NO (100 μM) for 30 min, and S-nitrosylated proteins were selected using organomercury columns followed by immunoblotting for RhoA (SNO-RhoA, top panel) versus total RhoA in cell lysates (total RhoA, bottom panel). The relative densitometry of SNO-RhoA vs total-RhoA is expressed as means ± S.E., *P < 0.05 versus control. (n = 3–6).