Highlights
-
•
We report on the application of Irreversible electroporation (IRE) on locally advanced pancreatic cancer (LAPC).
-
•
We report on a minimally invasive surgical approach supported by laparoscopic ultrasound.
-
•
We report on a novel technique the benefits of IRE with the advantages of laparoscopic surgery.
-
•
In medical literature is in our knowledge the first experience.
Keywords: Irreversible electroporation, IRE, Tumor, Pancreatic advanced tumor, Laparoscopy
Abstract
Introduction
Pancreatic cancer is one of the most lethal cancers worldwide, with 5-years survival rate as low as 6%. The majority of pancreatic cancer patients present locally advanced or metastatic disease at diagnosis. Typically, patients affected by locally advanced pancreatic cancer (LAPC) do not undergo radical surgery but are treated with focal ablative therapies. However, a high rate of morbidity due to the heat sink effect has limited the application of ablative techniques on a routine basis in LAPC patients. Irreversible electroporation (IRE) has proved to be a new method of LAPC ablation.
Presentation of the case
A 69-year-old woman affected by LAPC with good response to systemic chemotherapy with FOLFIRINOX and residual 35 mm mass in the neck of the pancreas underwent to IRE through a minimally invasive surgical approach under laparoscopic ultrasound guide. The post-operative course was uneventful and the patient was discharged after 5 days. Six months after surgery she had no evidence of distant or recurrent disease.
Discussion
IRE has previously shown promising results in the treatment of LAPC, with relatively acceptable morbidity rates and improvement of survival. We report on the application of IRE through a minimally invasive surgical approach supported by laparoscopic ultrasound.
Conclusion
In conclusion, we propose a novel technical approach that combines the benefits of IRE on the treatment of patients affected by LAPC with the advantages of laparoscopic surgery.
1. Introduction
Pancreatic cancer is one of the most frequent causes of cancer-related death worldwide [1,2]. In fact, despite the advances in diagnostic procedures and multimodal treatment pancreatic cancer patients present poor prognosis, with 5-year survival <6%. At presentation 40% of pancreatic cancer patients are affected by locally advanced disease or locally advanced pancreatic cancer (LAPC) [1]. In LAPC, tumor infiltrates adjacent organs and vessels including the portal vein, the celiac artery and the superior mesenteric artery. Patients affected by LAPC do not frequently undergo radical surgery.
Focal ablative therapies with different energy sources have been developed to treat locally advanced primary tumors of the liver, lung, pancreas, kidney or prostate or distant metastases [[3], [4], [5], [6], [7], [8]]. Targeted ablative options include cryotherapy, high intensity focused ultrasound, radiofrequency ablation (RFA) and microwave ablation.
All of these targeted ablative options are associated with a marked increase in treatment-related complications [[9], [10], [11], [12]]. The application of thermal ablative techniques to LAPC has been previously associated to high rates of morbidity, as a result of a heat sink effect [13,14]. In LAPC the beneficial effects of ablation is limited by the close proximity of critical vascular structures to the targeted tumor lesions. In fact, the primary mechanism of cell death induced by thermal ablative techniques is represented by a coagulative necrosis that involves also often surrounding structures.
Electroporation (IRE) has proved to be a new method of tumor ablation that activates energy-dependent biochemical mechanisms that promote apoptosis. In contrast with other thermal ablative options, IRE has been shown to produce larger perivascular tumor death while sparing tissue scaffolds [13,27]. The use of IRE in the treatment of LAPC has apparently shown promising results, with relatively acceptable morbidity rates and improvement of survival [26,30]. In this manuscript we report on the first application of IRE in the treatment of a LAPC patient through a minimally invasive surgical approach under laparoscopic ultrasound guide. On the basis of our experience, we propose a novel technical approach that combines the benefits of IRE on the treatment of patients affected by LAPC with the advantages of laparoscopic surgery. This case report was conducted, and is reported in accordance with the SCARE criteria [31].
2. Case report
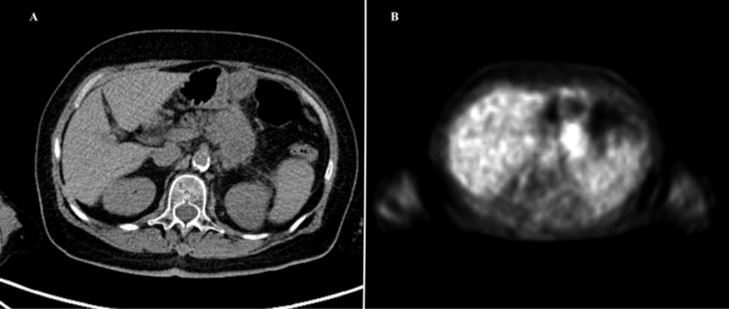
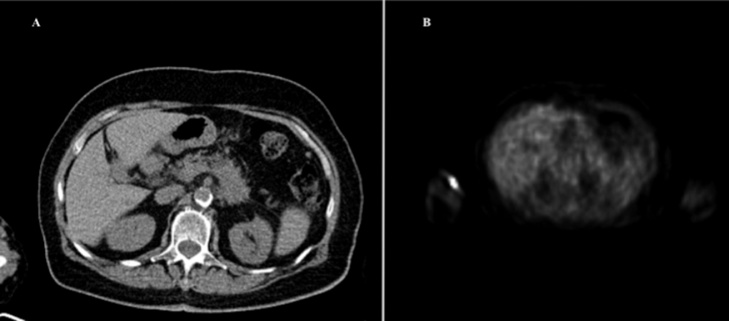
A 69-year-old woman affected by LAPC was admitted in our institute. At diagnosis CT identified a 35-mm pancreatic mass in the neck of the pancreas surrounding the superior mesenteric artery and splenic vein, without any signs of metastases. A systemic chemotherapy with FOLFIRINOX was performed. The PET/CT scan showed an area of high metabolic activity at the pancreatic body-tail (SUV 11.23) (Fig. 1). Subsequently, surgery was recommended by a multidisciplinary tumor board, in consideration of patient age, good response to chemotherapy and absence of distal metastasis.
Fig. 1.
Pre-operative PET/CT scan. A. CT scan shows a persistant pancreatic body-tail lesion. B. PET scan shows an area of high metabolic activity at the pancreatic body-tail (SUV 11.23).
Patient́s written consent was obtained for staging laparoscopy, open surgery and laparoscopic IRE. She was informed of the nature of IRE, of potential discomforts and risks.
2.1. Surgical technique
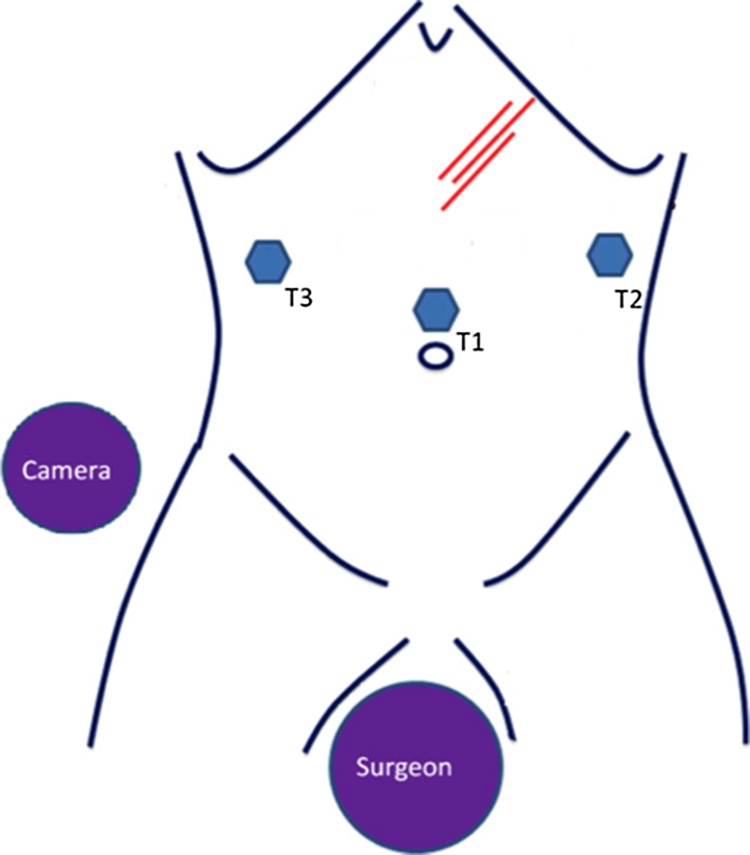
The patient was subjected to general anesthesia and, in addition, to a complete neuromuscular block to reduce muscle contractions due to IRE. The patient was placed supine with her legs abducted. The table was tilted in a mild reverse Trendelenburg position. The surgeon was standing between the patient legs, with the cameraman on her left side (Fig. 2). Pneumoperitoneum was obtained via the open Veress-assisted technique and a 30° scope was used [24]. Fig. 2 shows the position of the trocars.
Fig. 2.
Position of the surgical equipe, trocars and needles for IRE.
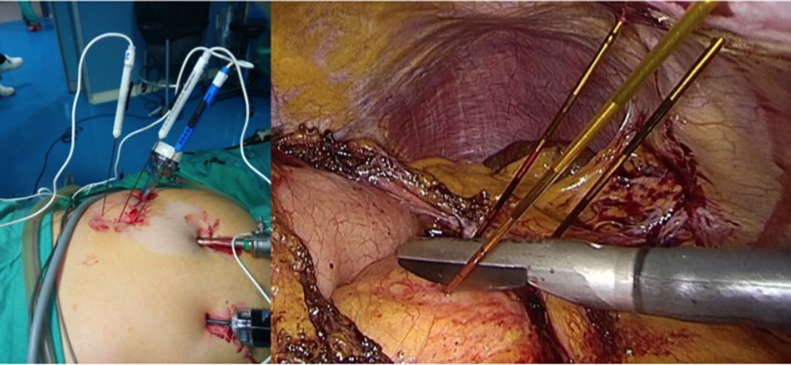
Staging laparoscopy was performed and no distant metastasis was identified. Under laparoscopic vision the gastro-colic ligament was opened near the middle of the stomach and was extended toward the infra-pyloric area using laparoscopic ultrasonic energy. In order to visualize the anterior surface of the pancreas the stomach was grasped and lifted cephalad. Intra-operative laparoscopic ultrasonography confirmed the impossibility to resect the mass with curative purpose (R0). Therefore, patient was treated by IRE (Fig. 3). IRE was performed laparoscopically following manufacture’s specifications and recommended parameters. Needles were inserted into the abdominal cavity through a coaxial stabilizing needles and guided into the pancreatic neoplasm by laparoscopic ultrasound (Fig. 4).
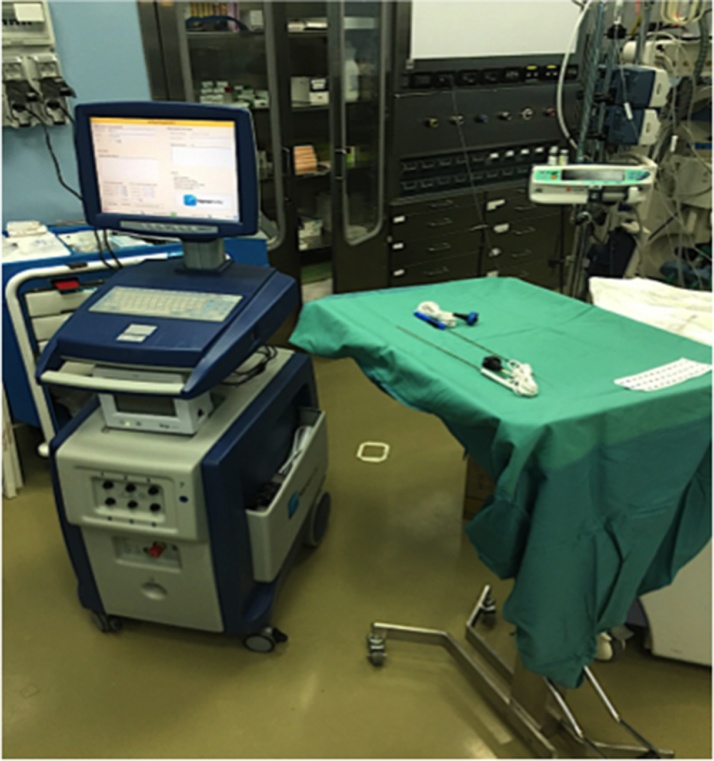
Fig. 3.
Irreversible electroporation machine, the NanoKnife® (Angio Dynamics, Queensburry, New York).
Fig. 4.
Percutaneous insertion of needles into the abdominal cavity and into the pancreatic neoplasm under laparoscopic ultrasound guide.
Needles were placed on both sides of the tumor to treat adjacent regions of the mass with 4 applications. Subsequently, ablation was performed and needles were removed under direct visual control to ensure hemostasis. Surgical time was 182 min. The post-operative course was uneventful and the patient was discharged after 5 days.
PET CT scan 6 months after surgery showed no significant increase in metabolic activity (SUV 3.42) (Fig. 5) at the pancreatic body tail.
Fig. 5.
Post-operative PET/CT scan at 6 months from surgery. Reduction of the metabolic activity at the pancreatic body tail.
3. Discussion
In this report we present a case on the application of irreversible electroporation for LAPC patients by a minimally invasive surgical approach, which made use of laparoscopic ultrasound for electrode positioning. To our knowledge this is the first reported case.
Pancreatic cancer is one of the most aggressive human malignancies. Patients with locally advanced cancer disease often present infiltration of adjacent structures such as the portal vein, the celiac trunk and/or the superior mesenteric vein [[25], [26], [27], [28], [29]].
In these cases, surgical resection may not be feasible and, consequently, the use of thermal ablative therapies such as RFA represents the only treatment with the intention of cure. However, RFA treatment of pancreatic cancer is characterized by a high rate of complications [26], since it may cause thermal injury to surrounding organs and structures. Recently, the use of IRE has been shown to improve survival in primarily unresectable solid tumors, including prostate, kidney, bone and/or liver cancer. IRE apparently represents an effective ablative therapy for patients with liver cancer and/or and LAPC. IRE is based on the principle of electroporation or electropermeabilization, whereby electric pulses are used to create defects in the cell membrane. These defects, defined “nano-pores” or “conductive pores”, spread to the whole plasmatic membrane, causing programmed cell death due to the inability of the cell to maintain homeostasis [15]. The genetically program activated by IRE into tumor cells prevents fibrosis, scaring and the disruption of tissue architecture. Macroscopically, this mechanism results in an absence of a heat sink that characterize necrotic cell death and, as a consequence, in a lower injury to adjacent vital structures [17,18].
Actually the commercial ablation device is set to produce high voltages, usually between 15,003000 V in pulses of 70,100 microseconds. It is of paramount importance to avoid cardiac arrhythmias during IRE administration. To this aim, during IRE treatment of LAPC patients, synchronization of IRE pulses with the cardiac rhythm is required [23].
Martin et al. reported on an improvement of overall and progressionfree survival in patients treated with IRE when compared with those who were treated with chemotherapy and/or radiation therapy alone [27]. In a recent review of the literature, Yeung et al. concluded that IRE was a potentially effective ablative therapy in a cohort of 114 patients affected by liver cancer [30]. However, the role of IRE as first–line regimen was not clearly defined [30].
As to the treatment of LAPC patients, IRE has been shown to be a promising approach, with relatively acceptable morbidity rates and improvement of survival. In these studies [18,27]. IRE was indicated for patients with tumor size ≤4 cm, after having received systemic therapy. IRE was administered with an approach of open surgery, which allowed precise probe placement under ultrasound guide.
In this manuscript we report on the application of IRE through a minimally invasive surgical approach supported by placing of probes by laparoscopic ultrasound. We propose a novel technical approach that combines the benefits of IRE for the treatment of patients affected by LAPC with the advantages of laparoscopic surgery.
Furthermore, it is our opinion that patients with a short life expectancy may highly benefit from minimally invasive surgery, resulting into better quality of life, minimization of complications and reduction of hospitalization times. Notably, it is advisable that IRE supported by laparoscopic ultrasound has to be performed by surgeons with skills in mininvasive pancreatic surgery and laparoscopic ultrasound who operate in high-throughput hospitals.
4. Conclusions
Our study shows that IRE supported by laparoscopic ultrasound is a promising treatment for patient affected by unresectable LAPC, though further studies on a more numerous cohort of patients are warranted to unambiguously define its pros and cons.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
Anything to declare.
Ethical approval
Ethical Approval was not required, it was a retrospective study.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”.
Authors provide assurance that the manuscript does not contain identifying characteristics of patient and alterations do not distort scientific meaning of this case report.
Author contribution
ET and MF conceived the study, participated in its design, drafted and revised the manuscript. They equally contributed.
AR conceived the study, participated in its design, drafted the manuscript.
AS drafted the manuscript.
RA drafted the manuscript.
LG drafted the manuscript.
PF drafted the manuscript.
DC participated in the design of the study and revised the manuscript.
FC conceived the study, operated the patients and revised the manuscript.
All authors have read and approved the final manuscript.
Registration of research studies
None.
Guarantor
Doctor Antonia Rizzuto is the Guarantor of the study. She conceived and conducted the study, had access to the data and controlled the decision to publish of the final manuscript previously approved by all authors.
Contributor Information
Ernesto Tartaglia, Email: ernesto.tartaglia@gmail.com.
Antonia Rizzuto, Email: arizzuto@unicz.it.
References
- 1.American Cancer Society . American Cancer Society; Atlanta: 2014. Cancer Facts & Figs. 2014. [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. International Agency for Research on Cancer; Lyon, France: 2014. GLOBOCAN 2012 v1.1, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] (Accessed May 24 2015) [Google Scholar]
- 3.Lencioni R., Cioni D., Della Pina C., Crocetti L. Hepatocellularcarcinoma: new options for image-guided ablation. J. Hepatobiliary Pancreat. Sci. 2010;17:399–403. doi: 10.1007/s00534-009-0233-0. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A., Abtin F., Shepard J.A. Image-guided ablative therapies for lung cancer. Radiol. Clin. North Am. 2012;50:975–999. doi: 10.1016/j.rcl.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Volpe A., Cadeddu J.A., Cestari A. Contemporarymanagementof small renal masses. Eur. Urol. 2011;60:501–515. doi: 10.1016/j.eururo.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 6.Olweny E.O., Cadeddu J.A. Novelmethodsforrenaltissueablation. Curr. Opin. Urol. 2012;22:379–384. doi: 10.1097/MOU.0b013e328355ecf5. [DOI] [PubMed] [Google Scholar]
- 7.Valerio M., Ahmed H.U., Emberton M. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur. Urol. 2014;66:732–751. doi: 10.1016/j.eururo.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Bos W., Muller B.G., Ehdaie B., Scardino P., de la Rosette J.J. What is still needed to make focal therapy an accepted segment of standard therapy? Curr. Opin. Urol. 2014;24:247–255. doi: 10.1097/MOU.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 9.Matsui Y., Nakagawa A., Kamiyama Y., Yamamoto K., Kubo N., Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14–20. doi: 10.1097/00006676-200001000-00002. PMID: 10630378. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y., Tang Z., Fang H., Gao S., Chen J., Wang Y., Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J. Surg. Oncol. 2006;94:392–395. doi: 10.1002/jso.20580. PMID: 16967436. [DOI] [PubMed] [Google Scholar]
- 11.Ierardi A.M., Lucchina N., Petrillo M., Floridi C., Piacentino F., Bacuzzi A., Fonio P., Fontana F., Fugazzola C., Brunese L., Carra ello G. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol. Med. 2014;119:483–498. doi: 10.1007/s11547-014-0417-9. PMID: 24981482. [DOI] [PubMed] [Google Scholar]
- 12.Rombouts S.J., Vogel J.A., van Santvoort H.C., van Lienden K.P., van Hillegersberg R., Busch O.R., Besselink M.G., Molenaar I.Q. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br. J. Surg. 2015;102:182–193. doi: 10.1002/bjs.9716. PMID: 25524417. [DOI] [PubMed] [Google Scholar]
- 13.Zorbas G., Samaras T. A study of the sink effect by blood vessels in radiofrequency ablation. Comput. Biol. Med. 2015;57:182–186. doi: 10.1016/j.compbiomed.2014.12.014. PMID: 25575184. [DOI] [PubMed] [Google Scholar]
- 14.Mann C.D., Metcalfe M.S., Lloyd D.M., Maddern G.J., Dennison A.R. The safety and efficacy of ablative techniques adjacent to the hepatic vasculature and biliary system. ANZ J. Surg. 2010;80:41–49. doi: 10.1111/j.1445-2197.2009.05174.x. PMID: 20575879. [DOI] [PubMed] [Google Scholar]
- 15.Wagstaff P.G., de Bruin D.M., Zondervan P.J. The efficacy and safety of irreversible electroporation for the ablation of renal masses: a pro- spective, human, in-vivo study protocol. BMC Cancer. 2015;15:165. doi: 10.1186/s12885-015-1189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang D.C., Reese T.S. Changes in membrane-structure induced by electroporation as revealed by rapid-freezing electron-microscopy. Biophys. J. 1990;58:1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bower M., Sherwood L., Li Y., Martin R. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J. Surg. Oncol. 2011;104:22–28. doi: 10.1002/jso.21899. PMID: 21360714. [DOI] [PubMed] [Google Scholar]
- 23.Thomson K.R., Cheung W., Ellis S.J. Investigation of the safety of irreversible electroporation in humans. J. Vasc. Interv. Radiol. 2011;22:611–621. doi: 10.1016/j.jvir.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Corcione F., Miranda L., Settembre A., Capasso P., Piccolboni D., Cusano D., Bakhtri M., Manzi F. Open Veress Assisted technique. Results in 2700 cases. Minerva Chir. 2007;62:443–446. [PubMed] [Google Scholar]
- 25.Yeo C.J. Pancreatic cancer −in brief. Curr. Probl. Cancer. 2002;26:170. doi: 10.1067/mcn.2002.129579. [DOI] [PubMed] [Google Scholar]
- 26.Pandya G.J., Shelat V.G. Radiofrequency ablation of pancreatic ductal adenocarcinoma: the past, the present and the future. World J. Gastrointest. Oncol. 2015;7:6–11. doi: 10.4251/wjgo.v7.i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin R.C., McFarland K., Ellis S., Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann. Surg. Oncol. 2013;20(Suppl. 3):S443–S449. doi: 10.1245/s10434-012-2736-1. PMID: 23128941. [DOI] [PubMed] [Google Scholar]
- 28.Månsson C., Bergenfeldt M., Brahmstaedt R., Karlson B.M., Nygren P., Nilsson A. Safety and preliminary efficacy of ultrasound-guided percutaneous irreversible electroporation for treatment of localized pancreatic cancer. Anticancer Res. 2014;34:289–293. PMID: 24403476. [PubMed] [Google Scholar]
- 29.Rizzuto A., Palaia I., Vescio G., Serra R., Malanga D., Sacco R. Multivisceral resection for occlusive colorectal cancer: is it justified? Int. J. Surg. 2016;33(September (Suppl. 1)):S142–S147. doi: 10.1016/j.ijsu.2016.06.021. Epub 2016 Jul 8. [DOI] [PubMed] [Google Scholar]
- 30.Yeung E.S., Chung M.W., Wong K., Wong C.Y., So E.C., Chan A.C. An update on irreversible electroporation of liver tumours. Hong Kong Med. J. 2014;20(August (4)):313–316. doi: 10.12809/hkmj134190. Epub 2014 Jun 6. Review. [DOI] [PubMed] [Google Scholar]
- 31.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34(October):180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]