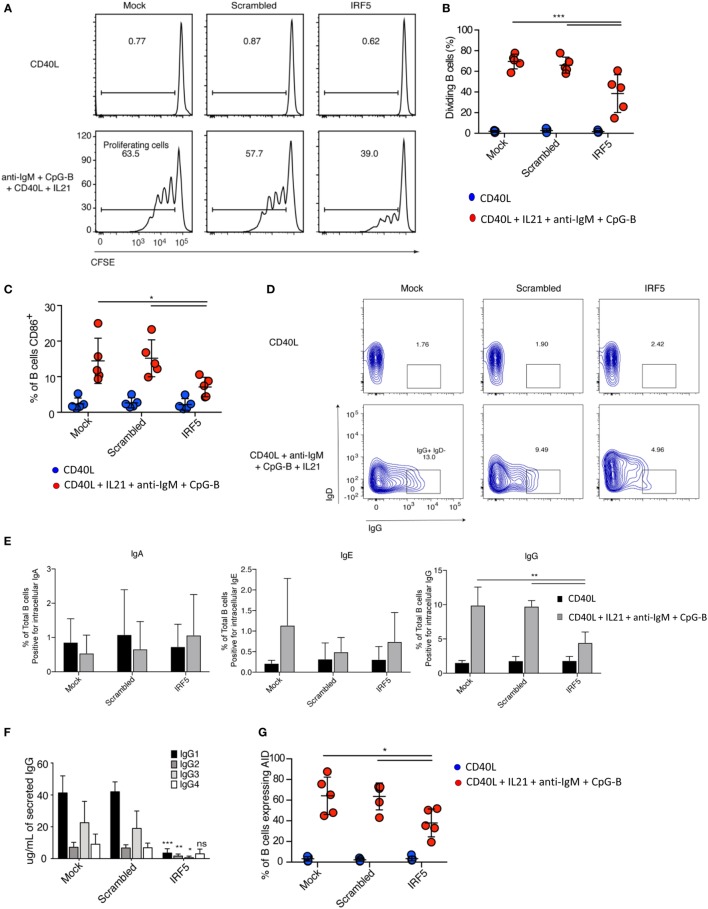
Figure 3.
Interferon regulatory factor 5 (IRF5) knockdown impairs B cell proliferation, activation, and immunoglobulin (Ig) G isotype secretion. (A) Representative flow cytometry histograms from cell proliferation assay as measured through dilution of the proliferation dye CFSE. Isolated primary naive B cells from a single donor were nucleofected with 500 nM of mock, scrambled or IRF5 siRNA and stimulated with either CD40 ligand (CD40L) or the combination of CD40L, IL21, anti-IgM, and CpG-B for 5 days. (B) Average percentage of proliferating B cells from (A) is shown (two-way ANOVA with Tukey’s post hoc test; n = 5 independent donors). (C) Average percentage of B cells expressing CD86. Similar to (A) except isolated naive B cells were nucleofected and then stimulated with CD40L, IL21, anti-IgM, and CpG-B for 24 h, then stained for surface CD86 (two-way ANOVA with Tukey’s post hoc test; n = 5 independent donors). (D) Representative contour plots from IgD−IgG+ B cells after gating on total CD19+CD20+ B cells. Isolated primary naive B cells were nucleofected with 500 nM of mock, scrambled or IRF5 siRNA and stimulated with either CD40L or the combination of CD40L, IL21, anti-IgM, and CpG-B for 7 days. (E) Quantification from (D) of Ig antibody class expression was determined by intracellular flow cytometery (one-way ANOVA with Tukey’s post hoc test; n = 5 independent donors). (F) Cell culture supernatants from (D) were used for ELISA to determine IgG isotype secretion. Average concentration of IgG isotype is shown (two-way ANOVA with Tukey’s post hoc test; n = 4 independent donors). (G) Frequency of activation-induced deaminase (AID) expression in B cells following nucleofection with 500 nM of mock, scrambled or IRF5 siRNA and stimulated with CD40L, IL21, anti-IgM, and CpG-B for 3 days. AID expression was determined by intracellular flow cytometry (one-way ANOVA with Tukey’s post hoc test; n = 5 independent donors). Error bars represent SD. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.