Abstract
Background
Current consensus statements maintain that endoscopic vein harvesting (EVH) should be standard care in coronary artery bypass graft surgery, but vein quality and clinical outcomes have been questioned. The VICO trial (Vein Integrity and Clinical Outcomes) was designed to assess the impact of different vein harvesting methods on vessel damage and whether this contributes to clinical outcomes after coronary artery bypass grafting.
Methods
In this single-center, randomized clinical trial, patients undergoing coronary artery bypass grafting with an internal mammary artery and with 1 to 4 vein grafts were recruited. All veins were harvested by a single experienced practitioner. We randomly allocated 300 patients into closed tunnel CO2 EVH (n=100), open tunnel CO2 EVH (n=100), and traditional open vein harvesting (n=100) groups. The primary end point was endothelial integrity and muscular damage of the harvested vein. Secondary end points included clinical outcomes (major adverse cardiac events), use of healthcare resources, and impact on health status (quality-adjusted life-years).
Results
The open vein harvesting group demonstrated marginally better endothelial integrity in random samples (85% versus 88% versus 93% for closed tunnel EVH, open tunnel EVH, and open vein harvesting; P<0.001). Closed tunnel EVH displayed the lowest longitudinal hypertrophy (1% versus 13.5% versus 3%; P=0.001). However, no differences in endothelial stretching were observed between groups (37% versus 37% versus 31%; P=0.62). Secondary clinical outcomes demonstrated no significant differences in composite major adverse cardiac event scores at each time point up to 48 months. The quality-adjusted life-year gain per patient was 0.11 (P<0.001) for closed tunnel EVH and 0.07 (P=0.003) for open tunnel EVH compared with open vein harvesting. The likelihood of being cost-effective, at a predefined threshold of £20 000 per quality-adjusted life-year gained, was 75% for closed tunnel EVH, 19% for open tunnel EVH, and 6% for open vein harvesting.
Conclusions
Our study demonstrates that harvesting techniques affect the integrity of different vein layers, albeit only slightly. Secondary outcomes suggest that histological findings do not directly contribute to major adverse cardiac event outcomes. Gains in health status were observed, and cost-effectiveness was better with closed tunnel EVH. High-level experience with endoscopic harvesting performed by a dedicated specialist practitioner gives optimal results comparable to those of open vein harvesting.
Clinical Trial Registration
URL: https://www.isrctn.com. International Standard Randomised Controlled Trial Registry Number: 91485426.
Keywords: coronary artery bypass, cost-benefit analysis, endothelium, treatment outcome, veins
Arterial conduits play a vital role in coronary artery bypass grafting (CABG) surgery because of their physical and functional properties. The internal mammary arteries are considered to be a gold standard conduit for bypass surgery because of their high patency and long-term survival rates.1 Only 4% to 12% patients receive bilateral internal mammary arteries in United States and European countries.2 The long saphenous vein remains the preferred conduit for multiple coronary artery bypass graft surgeries because of its long length, and endoscopic vein harvesting (EVH) has demonstrated reduced postoperative morbidity and improved patient satisfaction.3,4 Indeed, EVH is associated with markedly reduced scarring, diminished post-operative pain, greater patient mobility, and reduced inflammation.4 EVH also significantly reduces the likelihood of postoperative wound infections, potentially ameliorating the requirement for antibiotic use.5 Two EVH techniques exist: closed tunnel EVH (CT-EVH) and open tunnel EVH (OT-EVH), which differ on the basis of CO2 pressurization and instrumentation.
There is major debate about vein quality and long-term clinical outcomes after EVH, largely as a result of the findings of a major study6 that revealed poorer outcomes with EVH. However, this raised questions about the use of different systems (CT-EVH was used for the majority of EVH cases in that study), case selection, operator experience,7 and other comorbidities.8 Previous studies9–11 and systematic reviews12,13 have highlighted the need for an appropriately designed clinical trial to establish the effect of harvesting on vein integrity, downstream costs, and clinical outcomes.14 This was reinforced by the International Society of Minimally Invasive Cardiac Surgery3 and the National Institute for Health and Care Excellence.15,16 Many studies have compared EVH and open vein harvesting (OVH) in relation to wound-related complications and length of hospital stay, but no study has directly compared the histological and clinical outcomes of the 3 vein harvesting techniques.
We designed a prospective, single-center, 3-arm randomized study comparing vein damage and clinical outcomes between 2 types of EVH (CT and OT) and traditional OVH. A trial-based cost-effectiveness analysis was prospectively integrated within the study design to generate evidence of the cost-effectiveness of the vein harvesting techniques.
Methods
Study Design
The study was approved by the National Research Ethics Service Committee and was conducted following the principles of the Declaration of Helsinki and Good Clinical Practice. This study was undertaken at the University Hospital of South Manchester NHS Foundation Trust and was overseen by an external steering committee, clinical trial unit, and public patient involvement and safety monitoring board. The trial was registered on the Integrated Research Application System trial registry before patient recruitment started. We also registered the trial on the International Standard Randomised Controlled Trial Registry (91485426) in line with European Union regulation 536/2014. (The trial was submitted on April 30, 2014, and European Union regulation 536/2014 was released on May 27, 2014. The trial was fully registered on September 18, 2014.)
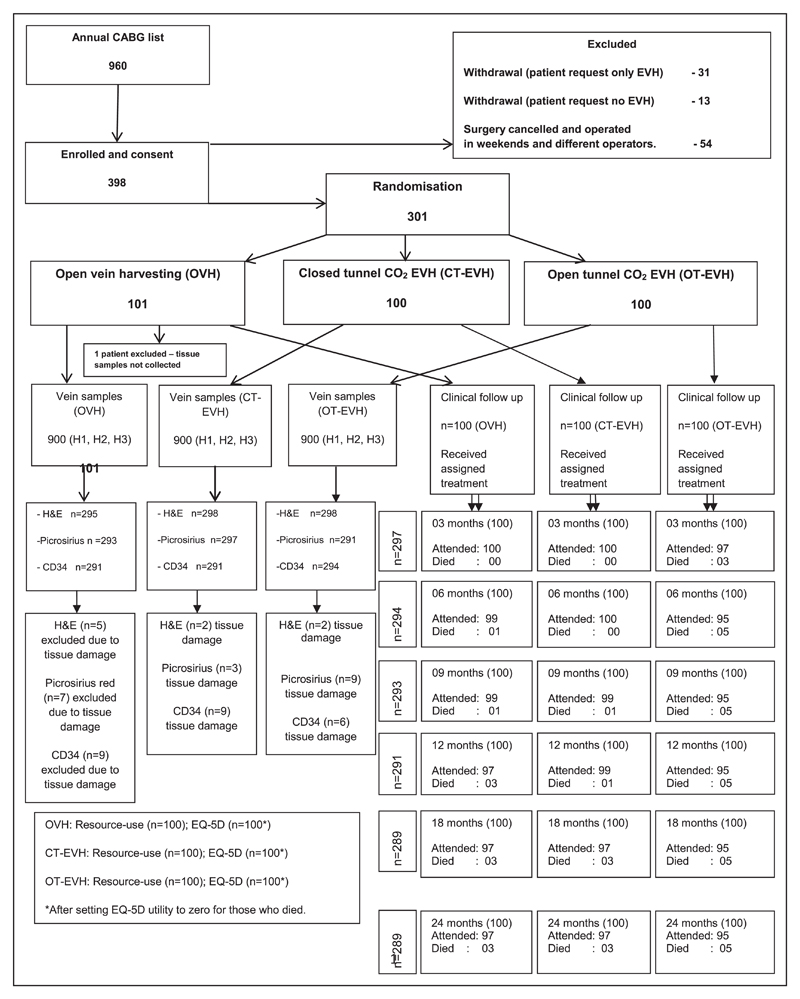
Patients who provided written informed consent were prospectively recruited between November 2011 and May 2015 from the cardiac waiting list (Figure 1). Patients who received single internal mammary artery and individual vein grafts1–4 by on-pump bypass were included (full study protocol describing recruitment, clinical and health economics data collection, method of histological scoring, and standard techniques included as Methods and diagrammatic overview provided in Figure I in the online-only Data Supplement). Exclusion criteria included emergency CABG, superficial long saphenous vein (<½ cm below the skin), varicose long saphenous vein, or small or thin legs (<7.5 cm diameter at the lower calf), determined via by an ultrasound Sonasite scans.4
Figure 1. CONSORT (Consolidated Standards of Reporting Trials) diagram demonstrating the detailed enrollment, treatment, and follow-up of the VICO trial (Vein Integrity and Clinical Outcomes) patients.
CABG indicates coronary artery bypass graft surgery; CT, closed tunnel; EQ-5D, EuroQol 5-dimensions questionnaire; EVH, endoscopic vein harvesting; H&E, hematoxylin and eosin; OT, open tunnel; and OVH, open vein harvesting.
Patients were randomized to 1 of 3 groups with a 1:1:1 allocation ratio. Computerized simple block randomization with random block sizes was performed by an independent statistician. Patient allocation was revealed to the practitioner once the patient was anaesthetized. Data-gathering researchers, the statistician, the health economist, and the histologist were completely blinded to the study group assignments.
Surgical Techniques
OVH and EVH were performed as previously described.4,17 All veins were harvested by an experienced surgical practitioner (>250 cases for each EVH technique and >2000 OVH cases). However, the CABG surgery was performed by 7 cardiac surgeons.
OVH: Control Group
According to normal practice, a long incision was made from ankle to thigh depending on the length of vein required for surgery. For the purpose of this study, the patients who required 2 lengths of vein had conduits harvested from just below the knee (≈9 cm). Patients who required 3 lengths of vein had the conduits harvested from 4 cm above the medial malleolus bone. The vein side branches were ligated with 4-0 Vicryl ties and titanium clips on both sides.4
CT-EVH: Intervention Group
The Maquet Vasoview Hemopro2 vein harvesting system, which involves a pressurized CO2 tunnel for vein dissection, was used. A 2- to 3-cm incision was made just above or below the knee (≈9 cm), depending on the length of vein (1, 2, or 3) required for surgery. The long saphenous vein was exposed and dissected with a West retractor and a Langenbeck retractor. The CO2 insufflator was set to 3 L/min with 0–mm Hg pressure. After completion of harvesting, patients received full heparinization followed by cardiopulmonary bypass. CT-EVH patients received 5000 U heparin before EVH to avoid intraluminal clot formation.18 A 30-mm, 0° endoscope with a sharp, clear dissecting cone on the tip was inserted through the skin incision. After 3 cm of anterior dissection, the balloon was inflated to seal the incision port. A minimal amount (10 mL) of trocar cuff air inflation was used to reduce the trauma to the vein. The vein was dissected from the surrounding tissues anteriorly and posteriorly until reaching the femoral junction in the groin. The vein side branches were ligated with 4-0 Vicryl ties and titanium clips on both sides.4
OT-EVH: Intervention Group
The Sorin ClearGlide vein harvesting system, which involves nonpressurized CO2 tunnel for vein dissection, was used. A 2- to 3-cm incision was made just above or below the knee (≈9 cm), depending on the number of vein lengths (1, 2, or 3) required for surgery. Initially, the long saphenous vein was exposed and dissected with a West retractor and a Langenbeck retractor. A 30-mm, 0° telescope with a ClearGlide dissecting retractor was introduced through the skin incision. The CO2 insufflator was set up at a continuous flow rate of 3 L/min and 0–mm Hg pressure. The vein was dissected from the surrounding tissue anteriorly and posteriorly until reaching the femoral junction in the groin. The vein side branches were ligated with 4-0 Vicryl ties and titanium clips on both sides. The small leg wound was closed in layers, and a dressing and pressure bandage were applied.4
Standardization for All 3 Group Techniques
The vein was harvested with surrounding fat and adventitial layers. The conduit was harvested 2 to 3 mm away from the main long saphenous vein.
All the branches were cut with at least 1 cm length whenever possible.
The vein was inflated with heparinized arterial blood with 10–mm Hg inflation pressure with a pressure control syringe.
The cardioplegia vein perfusion flow pressure through the vein was standardized to 70 mm Hg for all cases.
All patients requiring 3 lengths of vein had the conduits harvested from the ankle to the thigh. For patients who require 1 or 2 lengths, vein was harvested from just below or above the knee.
The measurements of partial pressure of arterial carbon dioxide and end-tidal carbon dioxide and any changes to the ventilator settings during the vein harvesting procedure were monitored and recorded for this study.
Histological Assessment
The 2700 vein samples were numerically coded to ensure laboratory blinding. Surgically undistended vein samples (n=900) were obtained proximally at the port of entry and coded H1. Distal vein samples (n=900) obtained after 10–mm Hg heparinized blood flush to check for leakages were coded H3. After vein grafting, a random sample was obtained from the remaining conduit and coded H2 (n=900). Therefore, H2 samples underwent all distension and manipulation as required for surgical preparation. Thus, these samples provide the best possible representation of the entire vein at different stages after harvesting that could be achieved given the logistics of the operation. These H2 samples were randomly given by the cardiac surgeons who were not told about the type of vein harvesting procedure to avoid any bias in relation to which segment was given for research purposes.
A computerized immunohistochemistry protocol was used to stain CD34 (a validated endothelial marker)19 of each vein sample from batch 1 (n=900; H1, H2, and H3). A validated scoring system was used to grade endothelial integrity20 (0% to 100% intact [positive staining]; Figure II in the online-only Data Supplement). The second batch of 900 vein samples was stained with Picrosirius red muscular and collagen stain (80-picrosirius red; Sigma-Aldrich Ltd, Dorset, UK) to assess structural damage in the muscular layers. We refined/modified the existing scoring system (full detailed scoring is given in Table V in the online-only Data Supplement) for simplicity, which was used to grade muscular hypertrophy (hypertrophy in this study means acute swelling rather than chronic process of the muscle injury), detachment, muscle migration on a scale of 0 to 3 (normal, mild, moderate, or severe; Figure III in the online-only Data Supplement). The final batch of 900 vein samples was stained with hematoxylin and eosin to assess endothelial stretching and detachment. Endothelial damage was graded on a scale of 0 to 3 (normal, mild, moderate, or severe) as detailed in Table VI in the online-only Data Supplement.19
All slides were scanned with a Pannoramic 250 slide scanning system. All histology images were scored by 5 independent assessors and validated by a consultant histopathologist.
Study Outcome Measures
The primary outcome measure was severity of histological damage to the vein conduits. The association between histological damage and predefined clinical outcomes was then assessed. Complete demographics, intraoperative details, incidence of wound infection, and general practitioner/district nurse visits were recorded.
The secondary end points included incidence of major adverse cardiac events (MACEs), use of healthcare resources, and impact on health status. A MACE was defined as repeat angina, breathlessness, myocardial ischemia/infarction, reintervention, stroke, and death. MACEs were determined by telephone interviews, clinic letters, and general practitioner and coroner reports at 3-month intervals until 12 months and then at 18, 24, 36, and 48 months. Only symptomatic MACE patients underwent cardiac magnetic resonance imaging scans, and angiograms were reviewed by an independent cardiologist and a cardiac surgeon.
An NHS and social services perspective was used for the scope of the collection of healthcare resources. All healthcare resources associated with treatment and follow-up care were recorded prospectively. Table I in the online-only Data Supplement provides a full list of healthcare use data collected and unit costs that were sourced from the procurement and finance department at the hospital and national databases when relevant for follow-up care.20,21 The vein harvesting procedure was microcosted, with the fixed cost of the vein harvesting equipment fully absorbed in each arm of the trial. The length of time within the operating theater required for vein harvesting was recorded and costed.
The impact on each individual’s health status was assessed at 3 and 12 months with the 3-level EuroQol 5-dimensions questionnaire (EQ-5D-3L), which has 5 domains (Mobility, Self-Care, Usual Activities, Pain and Discomfort, Anxiety and Depression) and 3 levels within each domain (no problems, some problems, severe problems). Using a published national tariff,22 we converted each completed EQ-5D-3L questionnaire for each patient into an index measure of health-related quality of life (HRQoL) on a scale from 1 (full health) to 0 (death). Health states with a HRQoL less than death were also included. Patients who died had an HRQoL of 0 inputted. Quality-adjusted life-years (QALYs) were calculated with the area under the curve method using the trapezoid rule and linear interpolation between the measures of HRQoL at the 2 time points. Because a 1-year time horizon was chosen, no discounting was applied to the cost or QALY data.
Power Calculation
To generate an accurate power calculation, we undertook a nonrandomized pilot study comparing the impact of the different vein harvesting techniques on endothelial integrity using 140 patients. From these pilot data, we calculated that 91 patients in each of the 3 groups (OVH, CT-EVH, and OT-EVH), that is, 273 in total, would provide 80% power to detect differences in the percentage with zero endothelial integrity of ≥20% (eg, 20% versus 40%) in this study. This calculation was based on a comparison of 2 groups using a simple χ2 test, with continuity correction at the 5% significance level. A recruitment strategy requiring a total of 300 patients with a 10% dropout rate was used.
Clinical outcomes in our pilot study demonstrated that 19% of CT-EVH patients experienced MACEs compared with 13% of OT-EVH patients (ie, only a 6% difference in incidence).
Statistical Analysis
All demographics were summarized as frequencies/percentages for categorical variables and means/median with SD/interquartile range (IQR) as appropriate for continuous variables. Endothelial integrity as determined by CD34 expression was presented as median percentage integrity, and other histological outcomes were presented as median scores and analyzed with the Kruskal-Wallis test. Composite and individual MACEs were analyzed at each time point with the χ2 test. All tests were performed as 2-tailed analyses, and values of P<0.05 were considered significant.
The χ2 test was used to compare how patients completed the EQ-5D-3L profile across the arms of the trial with values of P<0.05 considered to be significant. Incremental costs, incremental QALYs, and incremental net benefit at a decision maker’s threshold of £20 000 per QALY were calculated with regression analysis controlling for baseline disease severity measured by EQ-5D and the Canadian Cardiovascular Society grading score. For both costs and QALYs, different generalized linear models were tested to assess for fit to the data. The appropriate family for the generalized linear models was assessed with the Park test. The appropriate link for the generalized linear models was assessed with the Pearson correlation test, the Pregibon link test, and the modified Hosmer-Lemeshow test.23
For all regression models, a generalized linear model with an identity link and gaussian family, equivalent to ordinary least squares, was found to be the best specified and was used for the analysis. Statistical uncertainty was considered by using a nonparametric bootstrap method24 accommodating the correlation between costs and QALYs, and 1000 bootstrap replicates for each estimate were generated. Probabilities of cost-effectiveness were calculated by measuring the proportion of bootstrap replicates with a positive net benefit for a given cost-effectiveness threshold. A complete set of data were available for HRQoL and healthcare resource use, so no form of imputation was used.
Pilot Work
A pilot study was designed to determine study sample size for the primary end point and demonstrated that OT-EVH (n=70) better preserved conduit endothelium compared with CT-EVH (n=70; median, 65.0% versus 11.4%; P<0.001; Figure IV in the online-only Data Supplement). However, no significant differences were observed between groups for MACEs, including repeat angina (P=0.62), breathlessness (P=0.80), reintervention (P=1.00), myocardial infarction/ischemia (P=1.00), or mortality (P=0.44) up to 4 years after surgery (Table II in the online-only Data Supplement).
Results
Demographics
A total of 398 patients were enrolled, but 24.6% (98 patients) were excluded from the study on the basis of predefined exclusion criteria. Ninety-eight patients were excluded before the randomization of the patient allocation numbers, so they were not allocated into any specific trial groups (Figure 1). This method was used to avoid major dropout from the study. Our previous patient recruitment for the clinical trials indicated that patients change their participation in the trial or surgery schedule to accommodate emergency and urgent in-patient referrals. Thus, 301 patients underwent randomization, and there were no clinically relevant differences between groups (Table 1). However, 1 patient in OVH group was excluded after surgery because tissue samples were not collected. A higher body mass index, left main stem disease, and current smokers were observed in the CT-EVH group. Intraoperative variables were recorded, including surgical timings and number of veins required (Table III in the online-only Data Supplement).
Table 1. Demographic Data, Including Preoperative Comorbidities, Risk Factors, and Cardiac History.
| Demographic Variables | Group | P Value | ||
|---|---|---|---|---|
| OT-EVH (n=100) | OVH (n=100) | CT-EVH (n=100) | ||
| Age, y | 66.92±10.08 | 65.96±9.34 | 64.06±10.20 | 0.12 |
| M/F, n (%) | 82/18 (82.0/18.0) | 79/21 (79.0/21.0) | 79/21 (79.0/21.0) | 0.83 |
| Body mass index, kg/m2 | 27.77 (6.41) | 27.93 (5.45) | 28.78 (6.54) | 0.04 |
| Surgery, n (%) | ||||
| Elective | 46 (46.0) | 49 (49.0) | 41 (41.0) | 0.52 |
| Urgent | 54 (54.0) | 51 (51.0) | 59 (59.0) | |
| Diabetes mellitus, n (%) | ||||
| Diet controlled | 8 (8.0) | 6 (6.0) | 4 (4.0) | 0.49 |
| Tablet controlled | 21 (21.0) | 27 (27.0) | 22 (22.0) | 0.56 |
| Insulin controlled | 8 (8.0) | 11 (11.0) | 4 (4.0) | 0.18 |
| CCS score, n (%) | ||||
| I | 17 (17.0) | 17 (17.0) | 12 (12.0) | 0.69 |
| II | 25 (25.0) | 29 (29.0) | 33 (33.0) | |
| III | 45 (45.0) | 45 (45.0) | 46 (46.0) | |
| IV | 13 (13.0) | 9 (9.0) | 9 (9.0) | |
| New York Heart Association class, n (%) | ||||
| I | 27 (27.0) | 32 (32.0) | 40 (40.0) | 0.05 |
| II | 45 (45.0) | 35 (35.0) | 26 (26.0) | |
| III | 26 (26.0) | 25 (25.0) | 29 (29.0) | |
| IV | 2 (2.0) | 8 (8.0) | 5 (5.0) | |
| STEMI, n (%) | 18 (18.0) | 19 (19.0) | 29 (29.0) | 0.12 |
| NSTEMI, n (%) | 42 (42.0) | 48 (48.0) | 44 (44.0) | 0.69 |
| Previous PTCA, n (%) | 16 (16.0) | 12 (12.0) | 20 (20.0) | 0.30 |
| Previous MI, n (%) | 52 (52.0) | 43 (43.0) | 54 (54.0) | 0.25 |
| Multivessel disease, n (%) | 82 (82.0) | 81 (81.0) | 86 (86.0) | 0.61 |
| Left main stem disease, n (%) | 25 (25.0) | 25 (25.0) | 40 (40.0) | 0.03 |
| Hypertension, n (%) | 87 (87.0) | 83 (83.0) | 88 (88.0) | 0.56 |
| Smoking, n (%) | ||||
| Never smoked | 32 (32.0) | 33 (33.0) | 23 (23.0) | 0.03 |
| Previous smoker | 52 (52.0) | 54 (54.0) | 47 (47.0) | |
| Current smoker | 16 (16.0) | 13 (13.0) | 30 (30.0) | |
| Hypercholesterolemia, n (%) | 96 (96.0) | 90 (90.0) | 92 (92.0) | 0.25 |
| Peripheral vascular disease, n (%) | 19 (19.0) | 20 (20.0) | 21 (21.0) | 0.94 |
| Left ventricular ejection fraction, n (%) | ||||
| >50% | 74 (74.0) | 74 (74.0) | 72 (72.0) | 0.88 |
| 30%–50% | 21 (21.0) | 18 (18.0) | 22 (22.0) | |
| <30% | 5 (5.0) | 8 (8.0) | 6 (6.0) | |
Categorical variables are expressed as number (percent) and assessed by the χ2 test. Continuous variables are expressed as either mean±SD (parametric data) or median (interquartile range) (nonparametric data) and analyzed by ANOVA or independent-samples Kruskal-Wallis test, respectively. CCS indicates Canadian Cardiovascular Society; CT, closed tunnel; EVH, endoscopic vein harvesting; NSTEMI, non–ST-segment–elevated myocardial infarction; OT, open tunnel; OVH, open vein harvesting; PTCA, percutaneous coronary angioplasty; and STEMI, ST-segment–elevated myocardial infarction.
Primary Histological Outcomes
Endothelial Integrity: CD34
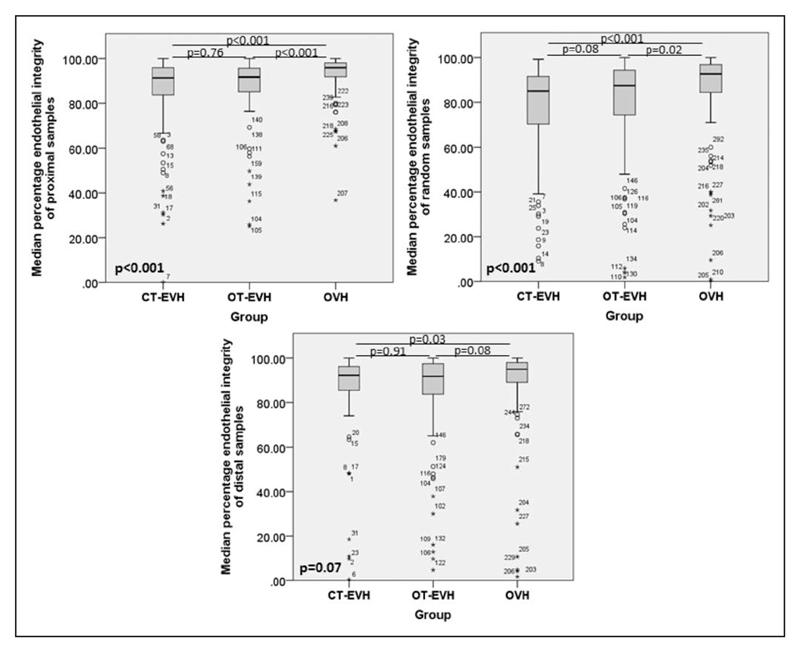
Endothelial integrity was better preserved in the OVH group in proximal samples compared with endoscopic techniques (median percentage integrity, 91.50 [IQR, 12.50] versus 91.63 [IQR, 10.56] versus 95.75 [IQR, 6.69] for CT-EVH versus OT-EVH versus OVH, respectively; P<0.001; Figure 2). Random samples from the OVH group displayed the greatest endothelial integrity compared with the other groups (85.25 [IQR, 21.13] versus 87.50 [IQR, 21.00] versus 92.71 [IQR, 13.13] for CT-EVH versus OT-EVH versus OVH, respectively; P<0.001; Figure 2). However, no statistical difference was observed in distal samples (92.25 [IQR, 10.88] versus 91.75 [IQR, 13.81] versus 95.38 [IQR, 9.25] for CT-EVH versus OT-EVH versus OVH, respectively; P=0.07; Figure 2).
Figure 2. Illustration of the median percentage endothelial integrity on proximal (H1), random (H2), and distal (H3) vein samples between the closed tunnel (CT) endoscopic vein harvesting (EVH), open tunnel (OT)-EVH, and open vein harvesting (OVH) groups.
Muscular Morphology: Picrosirius Red and Hematoxylin and Eosin
Endothelial stretching of proximal vein samples was greatest in the OT-EVH group (66.0%), followed by the CT-EVH group (61.0%), with least stretching in the OVH group (46.0%, P=0.01). No differences in endothelial stretching were observed between groups in distal (53.5% versus 51.5% versus 41.0% for OT-EVH, OVH, and CT-EVH, respectively; P=0.16) or random (37.4% versus 31.3% versus 36.7% for OT-EVH, OVH, and CT-EVH, respectively; P=0.62) samples. The level of endothelial detachment was consistent between groups in proximal (2% versus 3% versus 2% for OT-EVH, OVH, and CT-EVH; P=0.25), distal (4% versus 1% versus 6% for OT-EVH, OVH, and CT-EVH; P=0.63), and random (5% versus 2% versus 5% for OT-EVH, OVH, and CT-EVH; P=0.47) samples.
The circular muscle layer displayed greatest hypertrophy in proximal samples from the OT-EVH (65.6%) followed by the CT-EVH (45.0%) and OVH (14.3%, P<0.001) groups. A similar pattern was observed in distal (46.3% versus 24.2% versus 38.8% for OT-EVH, OVH, and CT-EVH; P<0.001) and random (35.4% versus 14.1% versus 31.3% for OT-EVH, OVH, and CT-EVH; P=0.01) samples. The longitudinal muscle layer displayed the greatest hypertrophy in proximal samples from the OT-EVH group (56.2%) compared with the OVH (5.1%) and CT-EVH (23.0%; P<0.001) groups. The greatest longitudinal hypertrophy was observed in distal samples from the OT-EVH group (26.3%) followed by the CT-EVH (8.2%) and OVH (1.0%, P<0.001) groups. Moreover, OT-EVH displayed greatest longitudinal hypertrophy in random samples (13.5%) compared with OVH (3.0%) and CT-EVH (1.0%, P=0.001).
Secondary Outcomes: Clinical Events and Cost-Effectiveness
Composite and individual MACE scores were analyzed in this study to avoid any varying definitions of composite outcomes. Kip et al15 suggested that authors should focus separately on safety and effectiveness outcomes.
Composite MACE Scores
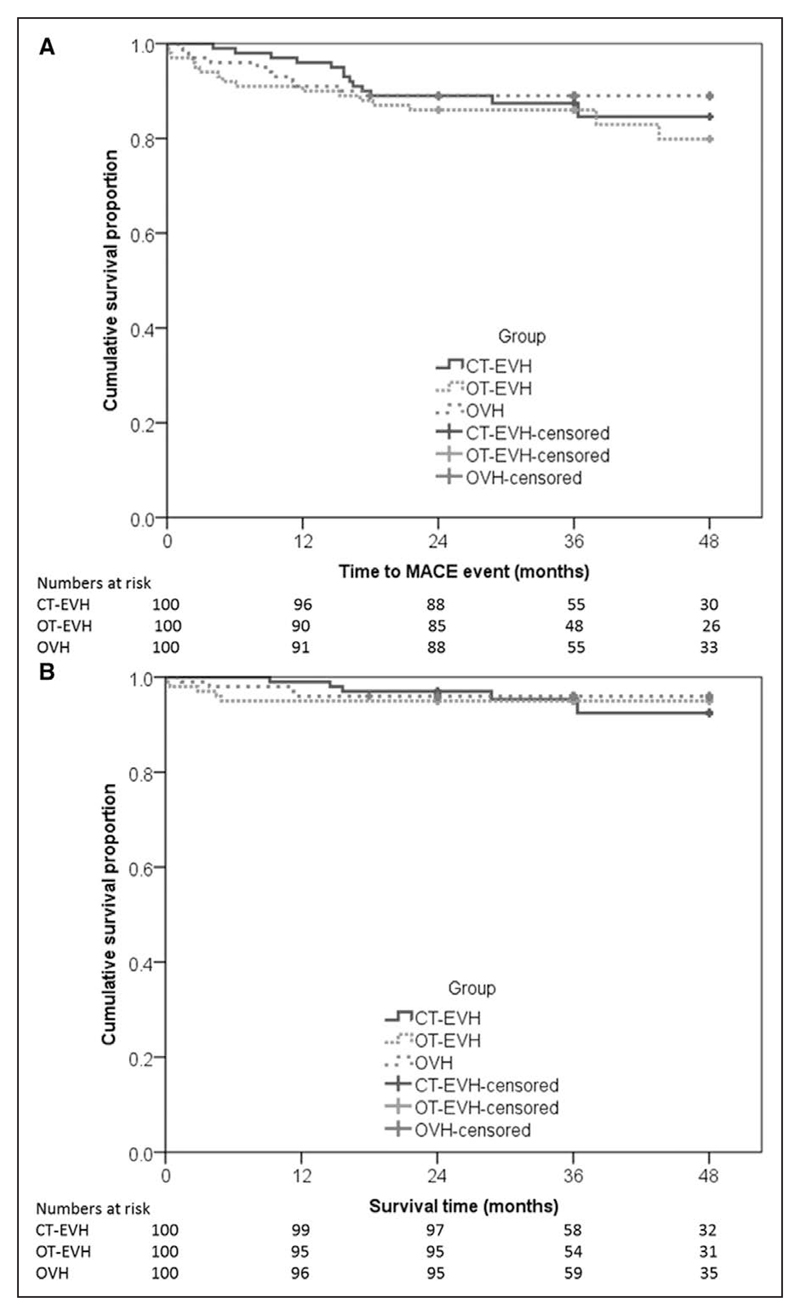
The incidence of composite MACEs was analyzed at each time point up to 48 months. No significant differences were observed between groups at any point (Figure 3A and Table IV in the online-only Data Supplement). Endothelial integrity did not differ between patients with and those without MACEs at 24 months (n=299) in proximal samples (median percentage integrity, 93.58 [IQR, 11.42] versus 92.33 [IQR, 7.54] for MACE-free and MACE-affected patients, respectively; P=0.48), distal samples (93.08 [IQR, 11.81] versus 96.25 [IQR, 11.50]; P=0.26), or random samples (88.75 [IQR, 18.56] versus 87.25 [IQR, 23.92]; P=0.64).
Statistically significant body mass index, left main stem disease, and number of current smokers were observed in CT-EVH group. A Cox proportional hazard model was considered. After adjustment for these variables, there does not appear to be a statistically significant relationship between the groups and time to first MACE (P=0.61; Table 2). However, these results should be interpreted cautiously because of small number of MACEs that occurred in this study.
Table 2. Cox Proportional Hazard Model for Major Adverse Cardiac Events.
| Hazards Ratio (95% CI) | P Value | |
|---|---|---|
| Unadjusted Cox PH model for MACEs | ||
| Variable: group | 0.56 | |
| CT-EVH | 1 (…) | |
| OT-EVH | 1.30 (0.62–2.70) | |
| OVH | 0.86 (0.39–1.93) | |
| Adjusted Cox PH model for MACEs | ||
| Variable: group | 0.61 | |
| CT-EVH | 1 (…) | |
| OT-EVH | 1.24 (0.58–2.66) | |
| OVH | 0.85 (0.37–1.95) | |
| Body mass index (per unit increase) | 0.96 (0.89–1.04) | 0.30 |
| Left main stem disease | 0.034 | |
| No | 1 (…) | |
| Yes | 2.00 (1.05–3.80) | |
| Smoking | 0.80 | |
| Never smoked | 1 (…) | |
| Previous smoker | 1.29 (0.61–2.72) | |
| Current smoker | 1.19 (0.46–3.03) | |
| New York Heart Association class | 0.003 | |
| I | 1 (…) | |
| II or higher | 4.91 (1.74–13.86) | |
After adjustment for body mass index, left main stem disease, smoking status, and New York Heart Association grade, the variables appear to be unbalanced between the randomization groups. There does not appear to be a statistically significant relationship between group and time to first MACE (P=0.61). The regression parameters and hazard ratios appear similar for the group variable in the unadjusted and adjusted Cox PH analyses, suggesting that the possible imbalances in the 4 other variables between the randomization groups do not affect its relationship with time to first MACE. These results should be interpreted cautiously because of the number of MACEs (totaling 33) and the number of parameters estimated in the adjusted Cox PH model, which was 7. CI indicates confidence interval; CT, closed tunnel; EVH, endoscopic vein harvesting; MACE, major adverse cardiac event; OT, open tunnel; OVH, open vein harvesting; and PH, proportional hazard.
Individual MACEs
The secondary outcomes demonstrated that no significant difference in MACEs was observed between groups other than slightly higher mortality at 3 and 6 months (P=0.05 and P=0.03, respectively) in the OT-EVH group (Figure 3B), although these deaths were not MACE-related mortalities. Atrial fibrillation occurred in 9 patients, and vein graft blockage was noted in 6 patients, although the incidence was not influenced by group (P=0.69 and P=0.42, respectively; Table 3). No statistically significant difference in MACE outcomes at each time point up to 48 months was observed between operating surgeons (P=0.76, P=0.78, P=0.26, P=0.23, and P=0.21, respectively).
Figure 3. Kaplan-Meier figure illustrating the (A) time to major adverse cardiac events and (B) cumulative survival at different time points until 48 months.
There does not appear to be a statistically significant difference between the groups in their MACE times (log-rank test, P=0.56) or their mortality and survival. CT indicates closed tunnel; EVH, endoscopic vein harvesting; OT, open tunnel; and OVH, open vein harvesting.
Table 3. Incidence of Postoperative Complications and Investigations.
| Variable | CT-EVH | OT-EVH | OVH | P Value |
|---|---|---|---|---|
| Chest wound numbness, n (%) | 57 (57.0) | 39 (40.2) | 52 (52.0) | 0.05 |
| Chest wound tenderness, n (%) | 49 (49.0) | 34 (35.1) | 42 (42.4) | 0.14 |
| Leg wound numbness, n (%) | 3 (3.0) | 10 (10.3) | 52 (52.5) | <0.001 |
| Leg wound tenderness, n (%) | 3 (3.0) | 7 (7.2) | 36 (36.4) | <0.001 |
| Arrhythmias, n (%) | 2 (2.0) | 3 (3.0) | 2 (2.1) | 0.87 |
| Atrial fibrillation, n (%) | 2 (2.0) | 3 (3.0) | 4 (4.1) | 0.69 |
| Ventricular fibrillation/tachycardia, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Pacemaker fitted, n (%) | 2 (2.0) | 2 (2.0) | 1 (1.0) | 0.83 |
| MRI performed, n (%) | 2 (2.0) | 6 (6.0) | 3 (3.1) | 0.30 |
| Angiography performed, n (%) | 2 (2.0) | 5 (5.2) | 5 (5.2) | 0.43 |
| Total MACEs, n (%) | 4 (4.0) | 6 (6.0) | 6 (6.0) | 0.77 |
| Cause of MACEs, n | ||||
| Vein not used because of small native coronary artery | 2* | 1 | 0 | … |
| Native artery disease | 1 | 1* | 2 | |
| Previous stent blocked | 2* | 1 | 1 | |
| LIMA blocked | 1* | 2* | 2 | |
| Vein graft insertional stenosis | 0 | 2* | 2 | |
| Vein graft blockage | 0 | 2* | 0 | |
CT indicates closed tunnel; EVH, endoscopic vein harvesting; LIMA, left internal mammary artery; MACE, major adverse cardiac event; MRI, magnetic resonance imaging; OT, open tunnel; and OVH, open vein harvesting. Postoperative complications and investigations carried out for the participants after coronary artery bypass graft surgery during the follow-up period are listed from the day of surgery until 48 months. In addition to the incidences, the detailed causes of MACEs are listed.
The same patient had multiple MACE causes.
Cost-Effectiveness Analyses
Vein harvesting costs for both the endoscopic approaches were higher than for the use of traditional OVH. The use of CT-EVH increased costs by £1180 (P<0.001), whereas OT-EVH increased costs by £981 (P<0.001) per patient over OVH. This increase in cost was due to one-off payments for the visual equipment needed to conduct the endoscopic extraction and an increase in variables costs required for each operation such as the need for additional disposable tubing and camera drapes. However, both endoscopic approaches led to lower downstream costs associated with follow-up care.
There was a reduction in postoperation costs for general practitioner visits, district nurse visits, and hospital stays (P<0.001). There was also a reduction in the cost for postoperative antibiotics use, cost for other medications, and the cost associated with “wound infection packages,” which includes readmission to the hospital, additional operating room costs for additional procedures, and vacuum-assisted closure therapy. Consequently, for follow-up care, CT-EVH led to a mean reduction in downstream costs of £814 (P=0.002) per person versus OVH, whereas OT-EVH led to a mean reduction of £598 (P=0.03). Overall, when the vein harvesting cost and downstream costs were combined, both EVH methods led to net cost increases over OVH, by £274 (P=0.34) for CT-EVH and £436 (P=0.16) for OT-EVH per patient, although neither was statistically significant.
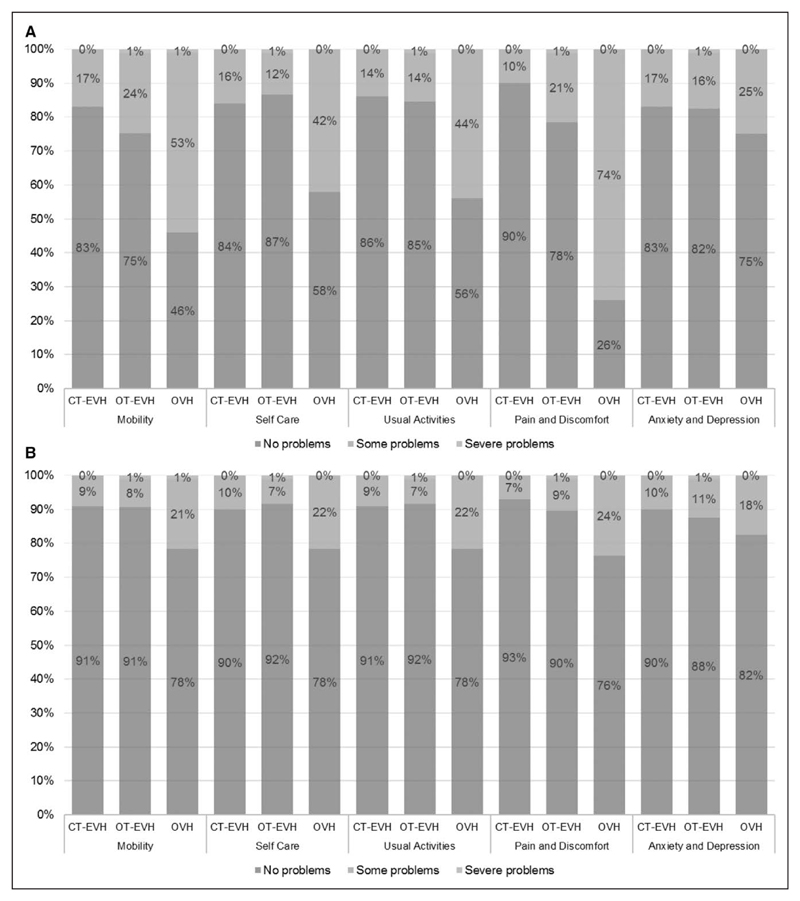
Both endoscopic approaches led to a marked improvement in HRQoL compared with the use of OVH. Figure 4A and 4B shows how patients completed the EQ-5D-3L at 3 and 12 months, respectively. At 3 months, in the Mobility, Self-Care, Usual Activities, and Pain and Discomfort domains, patients were more likely to report having some problems in the OVH arm compared with the endoscopic arms (P<0.001). At 12 months, patients were still more likely to report having some problems in the Self-Care (P=0.02), Usual Activities (P=0.01), and Pain and Discomfort (P=0.004) domains, but there was no significant effect for the Mobility domain (P=0.051). For the Anxiety and Depression domain, there was no difference between the arms at either 3 months (P=0.30) or 12 months (P=0.32).
Figure 4. How patients completed the 3-level EuroQol 5-dimensions questionnaire (percent selecting level) for each arm of the trial at (A) 3 months and (B) 12 months.
CT indicates closed tunnel; EVH, endoscopic vein harvesting; OT, open tunnel; and OVH, open vein harvesting.
Figure V in the online-only Data Supplement illustrates the impact on the EQ-5D-3L index after the EQ-5D-3L profiles have been weighted by the UK national tariff. The biggest difference in HRQoL occurs at 3 months, when patients in both endoscopic arms have higher HRQoL compared with patients in the OVH arm (P<0.001). At 12 months, there is an improvement in HRQoL across all arms, as well as some narrowing between the harvesting methods. At 12 months, both endoscopic approaches have higher mean values than OVH, which is statistically significant for CT-EVH versus OVH (P=0.004) but insignificant for OT-EVH (P=0.128). After calculation of the area under the curves, there was an increase in QALYs of 0.11 per patient (P=0.001) for the CT-EVH arm versus the OVH arm, whereas the OT-EVH arm had an increase in QALYs of 0.07 per patient (P<0.003) versus OVH.
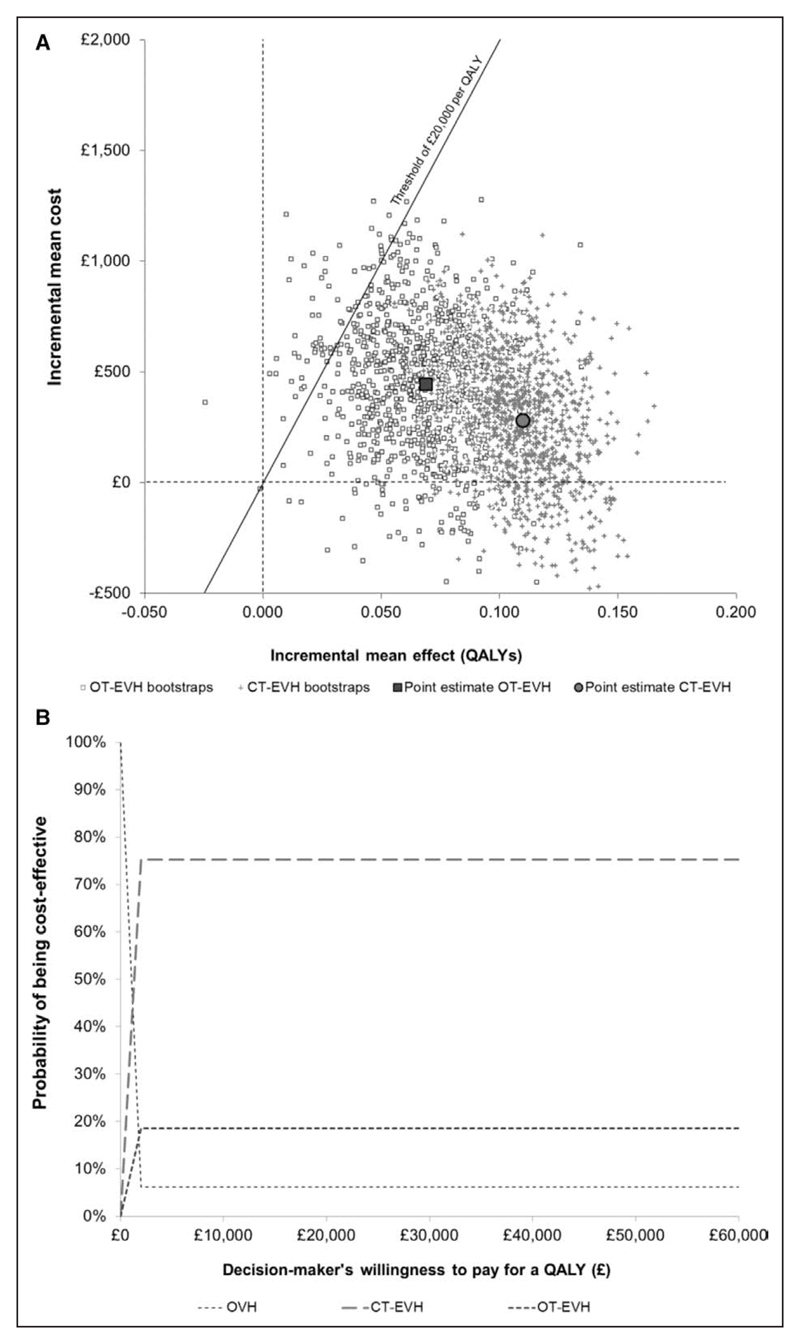
When we consider the costs and health benefits together to assess cost-effectiveness, CT-EVH had an incremental net benefit per patient of £1927 compared with OVH and a 75% likelihood of being cost-effective. This probability for cost-effectiveness is based on a decision maker being willing to spend an additional £20 000 for every additional QALY generated, called the cost-effectiveness threshold. OT-EVH had an incremental net benefit per patient of £950 versus OVH and a 19% likelihood of being cost-effective at a threshold of £20 000 per QALY. OVH had a low likelihood (6%) of being cost-effective (Figure 5A and 5B).
Figure 5. Cost-effectiveness.
A, Cost-effectiveness plane showing incremental costs and quality-adjusted life-years (QALYs) of closed tunnel (CT) endoscopic vein harvesting (EVH) and open tunnel (OT)-EVH vs open vein harvesting (OVH). Bootstrap replicates (n=2000) show the uncertainty with the larger points showing the point estimates. A cost-effectiveness threshold of £20 000 per QALY is presented. For a technology in the northeast quadrant, a cost-effective technology is one where the point estimate and a high proportion of bootstrap replicate falls below (southeast) the threshold line. B, Cost-effectiveness acceptability curve for OVH, CT-EVH, and OT-EVH plotted by calculating the proportion of bootstrap replicates falling below the cost-effectiveness threshold line as the threshold is varied. The typical threshold used by the National Institute for Health and Care Excellence is taken to be between £20 000 and £30 000 per QALY.
Safety and Clinical Relevance
At 24 months, composite MACEs were observed in 33 patients (OVH, 10 of 100; CT-EVH, 11 of 100; and OT-EVH, 12 of 100). A total of 289 patients survived, with noncardiovascular-associated mortality in 11 patients resulting from ischemic bowel, pneumonia, liver failure, and cancer. No mortality associated with cardiovascular events was observed. MACE repeat angina events (Table 3) were observed in 16 patients during the study period. Follow-up magnetic resonance imaging and angiogram evaluation in symptomatic patients concluded that angina was caused by native artery disease progression (4 of 16), vein graft insertional site stenosis (4 of 16), vein graft blocked (2 of 16), previous patent stent blocked (4 of 16), and left internal mammary artery insertional site stenosis (5 of 16). The vein conduits could not be grafted at the time of operation because of calcified/small coronaries in 3 of 16 patients. Multiple causes were observed in 5 patients.
Discussion
This is the first study with a direct head-to-head comparison of 2 EVH techniques with traditional OVH in relation to histological vein integrity and clinical outcomes. EVH has a number of important benefits and is associated with markedly improved postoperative patient satisfaction resulting from significantly less scarring, contributing to reduced pain and improved patient mobility compared with OVH. The smaller scar is also less likely to become infected, therefore necessitating less postoperative follow-up care. If graft patency can be maintained with EVH, then this would be a preferred option to OVH in suitable patients.
We report some vein injury in EVH compared with OVH (with loss of endothelial integrity, increased endothelial stretching, and muscle hypertrophy most severe in OT-EVH compared to CT-EVH and OVH). This study was powered for the primary outcome using our pilot work results to see the percentage of patients with zero endothelial injury, but we have not observed any conduits with zero endothelial integrity in any of the groups. Severe stretching and muscle migration have been associated with graft occlusion,25,26 yet only a small proportion of vein samples had severe intimal stretching in the OT-EVH group, and our subanalysis could not detect an association with MACEs.
In 2009, a major nonrandomized study concluded that EVH had higher rates of vein graft failure and mortality within 12 to 18 months after surgery.6 However, secondary outcomes from our randomized study demonstrated no statistically significant difference in composite or individual MACE scores with EVH, although a small sample size precluded firm conclusions. Furthermore, MACE scores did not correlate with vessel injury. This corroborates findings from previous studies describing positive clinical outcomes3,12,14 with both EVH and OVH and provides insight into the risk factors for MACEs.
Repeat angina and reintervention in patients in this study were due mainly to grafting technique and technical error,27,28 poor target-artery quality,11 progression of native coronary artery diseases,8 and previous stent blockage after CABG. Although the importance of grafting technique is highlighted by our findings, we did not observe significant intraoperator effects on MACE outcomes.
According to the International Society of Minimally Invasive Cardiac Surgery consensus statement,3 studies comparing OVH versus EVH have focused only on the cost of wound complications29 and readmissions and hospital stay,30 but no analysis of incremental cost-effectiveness has been conducted. Our study highlights that both EVH techniques led to modest net increases in cost compared with OVH during surgery. However, both EVH techniques substantially reduced postsurgery costs and improved patients’ HRQoL, leading to relatively large gains in QALYs compared with other technologies.31 The use of CT-EVH was associated with lower costs and better outcomes compared with OT-EVH. Therefore CT-EVH may represent the optimal cost-effective technique for vein harvesting.
Limitations
This study was designed to use a single experienced practitioner from 1 center to determine the impact of harvesting techniques. Different operators will inevitably introduce variability in surgical skills, which could confound a true comparison. The practitioner had experience of >2000 OVH cases but only 250 EVH cases, and this may have implications on surgical timing, quality of the OVH vein conduit, and postoperative complications, which need to be taken into consideration in the interpretation of the data. In addition, not all study participants underwent routine angiogram or cardiac magnetic resonance imaging scans because of ethical or financial restrictions within the NHS and risks involved as a result of patient age. The present study is underpowered to detect small differences in clinical outcomes because >1000 patients would be required in each arm, which would not be possible for a single-center study. However, this study was designed with graft histology as the primary outcome because this has been understudied to date and is an important area. For these purposes, a single-center, single-practitioner model was most appropriate. The principal reason for using a sole operator for this study is to minimize the incidence of practitioner skill error. In addition, we performed comparisons of MACE incidence at multiple time points, which could increase the likelihood of type 1 error and of obtaining statistically significant results by chance. However, we did not detect statistical differences in individual MACEs at any time point, so type 1 error did not alter our conclusions.
Conclusions
Our study demonstrated that EVH causes minimal damage to the layers of the vein. However, the small sample size in this study makes it difficult to conclude what impact this injury has on clinical outcomes with a large sample size. EVH also provides better HRQoL and QALYs and is more cost-effective than OVH after CABG surgery. Therefore, EVH can be used for vein harvesting safely with appropriate patient selection, the appropriate equipment, and better structured training of future practitioners. This study provides a base for future multicenter studies and clarifies that histological damage is minimal when practitioners are experienced.
Supplementary Material
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.117.028261/-/DC1.
Clinical Perspective.
What Is New?
The VICO trial (Vein Integrity and Clinical Outcomes) is the first study to directly evaluate the impact of minimally invasive and open vein harvesting techniques on the collective outcomes of endothelial integrity of the graft, clinical outcomes, health-related quality of life, and cost-effectiveness.
The study aimed to determine whether vein damage during harvesting contributed to outcomes after surgery. A single-center, sole-operator study was selected to minimize the incidence of practitioner skill error because this could markedly impair the validity of any findings between endoscopic vein harvesting methods.
What Are the Clinical Implications?
This study demonstrates that endoscopic vein harvesting induces minimal damage to vessel integrity, yet there is no direct correlation with clinical outcomes in a small sample size.
In addition, it highlights that endoscopic vein harvesting is likely to be cost-effective, reduces post-surgery costs, and improves patients’ health-related quality of life.
Our data support the use of endoscopic vein harvesting techniques as a routine care procedure for coronary artery bypass graft surgery in selected patients.
Practitioner experience is important in ensuring conduit quality, as demonstrated by the difference between our pilot and randomized study data.
Acknowledgments
The authors acknowledge Peter Walker, Dr Roger Meadows, Dr Peter March (University of Manchester), Dr Paul Bishop (consultant histopathologist), Dawn Clarke, and Catherine McNulty (University Hospital of South Manchester) for histological support. They acknowledge the consultant cardiothoracic surgeons who performed the surgery (Paul Waterworth, Mark T Jones, Tim Hooper, John Carey, Isaac Kadir, James Barnard) and surgical care practitioners (Janesh Nair, Nehru Devan). Statistical input was provided by Dr Philip Foden at the University of Manchester. The authors extend special thanks to all research students and researchers involved in this study.
Sources of Funding
Dr Krishnamoorthy is funded by a National Institute of Health Research, Clinical Doctoral Research Fellowship, England. This article presents independent research funded by the National Institute of Health Research. The views expressed are those of the authors and not necessarily those of the NHS, the National Institute of Health Research, or the Department of Health.
Footnotes
Disclosures
None.
Footnotes
Circulation is available at http://circ.ahajournals.org.
Contributor Information
Bhuvaneswari Krishnamoorthy, Departments of Cardiothoracic Surgery, University Hospital of South Manchester NHS Foundation Trust, United Kingdom; Manchester Collaborative Centre for Inflammation Research, Faculty of Biology, Medicine and Health, University of Manchester, United Kingdom; Faculty of Health and Social Care, Edge Hill University, Ormskirk, Lancashire, United Kingdom.
William R. Critchley, Manchester Collaborative Centre for Inflammation Research, Faculty of Biology, Medicine and Health, University of Manchester, United Kingdom.
Alexander J. Thompson, Manchester Centre for Health Economics, University of Manchester, United Kingdom.
Katherine Payne, Manchester Centre for Health Economics, University of Manchester, United Kingdom.
Julie Morris, Medical Statistics, University Hospital of South Manchester NHS Foundation Trust, United Kingdom.
Rajamiyer V. Venkateswaran, Departments of Cardiothoracic Surgery, University Hospital of South Manchester NHS Foundation Trust, United Kingdom.
Ann L. Caress, School of Nursing and Midwifery, University of Manchester, United Kingdom.
Dr James E. Fildes, Manchester Collaborative Centre for Inflammation Research, Faculty of Biology, Medicine and Health, University of Manchester, United Kingdom.
Prof Nizar Yonan, Departments of Cardiothoracic Surgery, University Hospital of South Manchester NHS Foundation Trust, United Kingdom.
References
- 1.Cameron A, Davis KB, Green G, Schaff HV. Coronary bypass surgery with internal-thoracic-artery grafts: effects on survival over a 15-year period. N Engl J Med. 1996;334:216–219. doi: 10.1056/NEJM199601253340402. [DOI] [PubMed] [Google Scholar]
- 2.Glineur D, Etienne PY, Kuschner CE, Shaw RE, Ferrari G, Rioux N, Papadatos S, Brizzio M, Mindich B, Zapolanski A, Grau JB. Bilateral internal mammary artery Y construct with multiple sequential grafting improves survival compared to bilateral internal mammary artery with additional vein grafts: 10-year experience at 2 different institutions†. Eur J Cardiothorac Surg. 2017;51:368–375. doi: 10.1093/ejcts/ezw282. [DOI] [PubMed] [Google Scholar]
- 3.Allen K, Cheng D, Cohn W, Connolly M, Edgerton J, Falk V, Martin J, Ohtsuka T, Vitali R. Endoscopic vascular harvest in coronary artery bypass grafting surgery: a consensus statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) 2005. Innovations (Phila) 2005;1:51–60. doi: 10.1097/01.gim.0000196315.32179.82. [DOI] [PubMed] [Google Scholar]
- 4.Krishnamoorthy B, Critchley WR, Glover AT, Nair J, Jones MT, Waterworth PD, Fildes JE, Yonan N. A randomized study comparing three groups of vein harvesting methods for coronary artery bypass grafting: endoscopic harvest versus standard bridging and open techniques. Interact Cardiovasc Thorac Surg. 2012;15:224–228. doi: 10.1093/icvts/ivs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yun KL, Wu Y, Aharonian V, Mansukhani P, Pfeffer TA, Sintek CF, Kochamba GS, Grunkemeier G, Khonsari S. Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: six-month patency rates. J Thorac Cardiovasc Surg. 2005;129:496–503. doi: 10.1016/j.jtcvs.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 6.Lopes RD, Hafley GE, Allen KB, Ferguson TB, Peterson ED, Harrington RA, Mehta RH, Gibson CM, Mack MJ, Kouchoukos NT, Califf RM, et al. Endoscopic versus open vein-graft harvesting in coronaryartery bypass surgery. N Engl J Med. 2009;361:235–244. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 7.Bisleri G, Muneretto C. Letter by Bisleri and Muneretto regarding article, “Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV”. Circulation. 2015;132:e28. doi: 10.1161/CIRCULATIONAHA.114.013672. [DOI] [PubMed] [Google Scholar]
- 8.Sabik JF, 3rd, Blackstone EH, Gillinov AM, Smedira NG, Lytle BW. Occurrence and risk factors for reintervention after coronary artery bypass grafting. Circulation. 2006;114(suppl):I454–I460. doi: 10.1161/CIRCULATIONAHA.105.001149. [DOI] [PubMed] [Google Scholar]
- 9.Chernyavskiy A, Volkov A, Lavrenyuk O, Terekhov I, Kareva Y. Comparative results of endoscopic and open methods of vein harvesting for coronary artery bypass grafting: a prospective randomized parallel-group trial. J Cardiothorac Surg. 2015;10:163. doi: 10.1186/s13019-015-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Diepen S, Brennan JM, Hafley GE, Reyes EM, Allen KB, Ferguson TB, Peterson ED, Williams JB, Gibson CM, Mack MJ, Kouchoukos NT, et al. Endoscopic harvesting device type and outcomes in patients undergoing coronary artery bypass surgery. Ann Surg. 2014;260:402–408. doi: 10.1097/SLA.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess CN, Lopes RD, Gibson CM, Hager R, Wojdyla DM, Englum BR, Mack MJ, Califf RM, Kouchoukos NT, Peterson ED, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation. 2014;130:1445–1451. doi: 10.1161/CIRCULATIONAHA.113.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sastry P, Rivinius R, Harvey R, Parker RA, Rahm AK, Thomas D, Nair S, Large SR. The influence of endoscopic vein harvesting on outcomes after coronary bypass grafting: a meta-analysis of 267,525 patients. Eur J Cardiothorac Surg. 2013;44:980–989. doi: 10.1093/ejcts/ezt121. [DOI] [PubMed] [Google Scholar]
- 13.Deppe AC, Liakopoulos OJ, Choi YH, Slottosch I, Kuhn EW, Scherner M, Stange S, Wahlers T. Endoscopic vein harvesting for coronary artery bypass grafting: a systematic review with meta-analysis of 27,789 patients. J Surg Res. 2013;180:114–124. doi: 10.1016/j.jss.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Markar SR, Kutty R, Edmonds L, Sadat U, Nair S. A meta-analysis of minimally invasive versus traditional open vein harvest technique for coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2010;10:266–270. doi: 10.1510/icvts.2009.222430. [DOI] [PubMed] [Google Scholar]
- 15.Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:701–707. doi: 10.1016/j.jacc.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Barnard JB, Keenan DJ, National Institute for Health and Clinical Endoscopic saphenous vein harvesting for coronary artery bypass grafts: NICE guidance. Heart. 2011;97:327–329. doi: 10.1136/hrt.2010.209668. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamoorthy B, Critchley WR, Bhinda P, Crockett J, John A, Bridgewater BJ, Waterworth PD, Fildes J, Yonan N. Does the introduction of a comprehensive structured training programme for endoscopic vein harvesting improve conduit quality? A multicentre pilot study. Interact Cardiovasc Thorac Surg. 2015;20:186–193. doi: 10.1093/icvts/ivu354. [DOI] [PubMed] [Google Scholar]
- 18.Brown EN, Kon ZN, Tran R, Burris NS, Gu J, Laird P, Brazio PS, Kallam S, Schwartz K, Bechtel L, Joshi A, et al. Strategies to reduce intraluminal clot formation in endoscopically harvested saphenous veins. J Thorac Cardiovasc Surg. 2007;134:1259–1265. doi: 10.1016/j.jtcvs.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashmi SF, Krishnamoorthy B, Critchley WR, Walker P, Bishop PW, Venkateswaran RV, Fildes JE, Yonan N. Histological and immunohistochemical evaluation of human saphenous vein harvested by endoscopic and open conventional methods. Interact Cardiovasc Thorac Surg. 2015;20:178–185. doi: 10.1093/icvts/ivu359. [DOI] [PubMed] [Google Scholar]
- 20.Hwang HY, Kim MA, Seo JW, Kim KB. Endothelial preservation of the minimally manipulated saphenous vein composite graft: histologic and immunohistochemical study. J Thorac Cardiovasc Surg. 2012;144:690–696. doi: 10.1016/j.jtcvs.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 21.National Schedule of Reference Costs: 2014–2015. London, UK: Department of Health; 2015. [Google Scholar]
- 22.Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: results from a UK general population survey. Discussion Paper No. 138. 1995.
- 23.Glick HA, D J, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. London, UK: Oxford University Press; 2007. [Google Scholar]
- 24.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6:327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Kanellaki-Kyparissi M, Kouzi-Koliakou K, Marinov G, Knyazev V. Histological study of arterial and venous grafts before their use in aortocoronary bypass surgery. Hellenic J Cardiol. 2005;46:21–30. [PubMed] [Google Scholar]
- 26.Wali MA, Eid RA. Intimal changes in varicose veins: an ultrastructural study. J Smooth Muscle Res. 2002;38:63–74. doi: 10.1540/jsmr.38.63. [DOI] [PubMed] [Google Scholar]
- 27.Shah DM, Darling RC, 3rd, Chang BB, Fitzgerald KM, Paty PS, Leather RP. Long-term results of in situ saphenous vein bypass: analysis of 2058 cases. Ann Surg. 1995;222:438–446. doi: 10.1097/00000658-199510000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnamoorthy B, Critchley WR, Venkateswaran RV, Barnard J, Caress A, Fildes JE, Yonan N. A comprehensive review on learning curve associated problems in endoscopic vein harvesting and the requirement for a standardised training programme. J Cardiothorac Surg. 2016;11:45. doi: 10.1186/s13019-016-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandt CP, Greene GC, Pollard TR, Hall WC, Bufkin BL, Briggs RM, Harville LE, Maggart ML, Ware RE. Review of efforts to decrease costly leg wound complications in the Medicare population following coronary revascularization. Heart Surg Forum. 2003;6:258–263. [PubMed] [Google Scholar]
- 30.Puskas JD, Wright CE, Miller PK, Anderson TE, Gott JP, Brown WM, 3rd, Guyton RA. A randomized trial of endoscopic versus open saphenous vein harvest in coronary bypass surgery. Ann Thorac Surg. 1999;68:1509–1512. doi: 10.1016/s0003-4975(99)00952-2. [DOI] [PubMed] [Google Scholar]
- 31.Wisløff T, Hagen G, Hamidi V, Movik E, Klemp M, Olsen JA. Estimating QALY gains in applied studies: a review of cost-utility analyses published in 2010. Pharmacoeconomics. 2014;32:367–375. doi: 10.1007/s40273-014-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.